Ribonuclease inhibitor
From Proteopedia
| Line 4: | Line 4: | ||
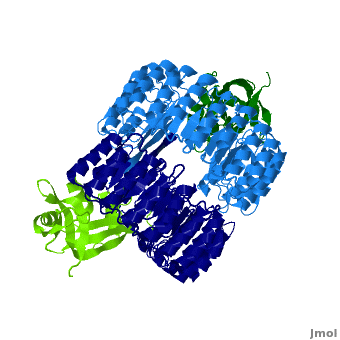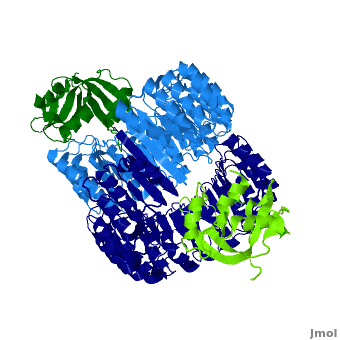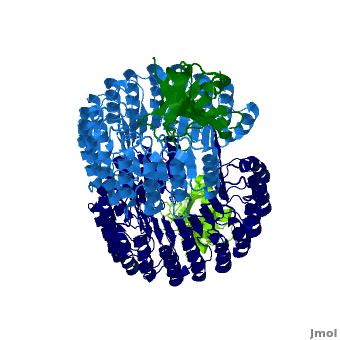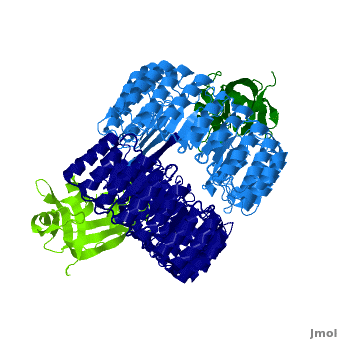
| - | <structuresection load='1z7x' size='500' frame='true' align='right' caption='hRI(blue) complexed with RNase 1(green) ' scene='Ribonuclease_inhibitor/1z7x/3' > | + | <structuresection load='1z7x' size='500' frame='true' align='right' caption='hRI(blue) complexed with RNase 1(green)' scene='Ribonuclease_inhibitor/1z7x/3' > |
| - | + | ||
| - | + | ||
| - | + | ||
==Interactions between hRI and RNase 1== | ==Interactions between hRI and RNase 1== | ||
Arg39 and Arg91 are proposed to be “electrostatic targeting residues” a term used by Johnson et. al to define residues that push the formation of protein complexes<ref>PMID:17350650</ref>. Mutagenic studies that changed Arg39 and Arg91 to leucines strongly affected the association rate of the complex. As shown, <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> and <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> form multiple hydrogen bonds to hRI, keeping the RNase in place, allowing the formation of salt bridges that further lock hRI and RNase together. <ref>PMID:17350650</ref> | Arg39 and Arg91 are proposed to be “electrostatic targeting residues” a term used by Johnson et. al to define residues that push the formation of protein complexes<ref>PMID:17350650</ref>. Mutagenic studies that changed Arg39 and Arg91 to leucines strongly affected the association rate of the complex. As shown, <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> and <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> form multiple hydrogen bonds to hRI, keeping the RNase in place, allowing the formation of salt bridges that further lock hRI and RNase together. <ref>PMID:17350650</ref> | ||
Revision as of 03:17, 9 November 2011
Ribonuclease inhibitors (RI) are a family of large (~450 residues, ~49 kDa), acidic (pI ~4.7), proteins that catalyze the degradation of ribonucleases. Human RI(hRI) is a major cellular protein, comprising ~0.1% of all cellular protein by weight. [1]
Ribonucleases (RNase) are enzymes that degrade RNA and are often cytotoxic which gives them chemotherapeutic properties. However, when bound to an RI they are no longer functional. Understanding the mechanism through which RI identifies and binds to RNases will allow scientists to design/modify RNases to evade hRI. In fact, one drug, Onconase (ONC), a ribonuclease from the Northern Leopard Frog (Rana pipiens), is now in Phase III clinical trials as a cancer chemotherapeutic agent [2].
| |||||||||||
3d structures
References
- ↑ Shapiro R. Cytoplasmic ribonuclease inhibitor. Methods Enzymol. 2001;341:611-28. PMID:11582809
- ↑ Zwolinska M, Smolewski P. [Onconase: a ribonuclease with antitumor activity]. Postepy Hig Med Dosw (Online). 2010 Feb 19;64:58-66. PMID:20173221
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
- ↑ Kobe B, Deisenhofer J. Mechanism of ribonuclease inhibition by ribonuclease inhibitor protein based on the crystal structure of its complex with ribonuclease A. J Mol Biol. 1996 Dec 20;264(5):1028-43. PMID:9000628 doi:http://dx.doi.org/10.1006/jmbi.1996.0694
Proteopedia Page Contributors and Editors (what is this?)
Abe Weintraub, Michal Harel, Alexander Berchansky, Joel L. Sussman