Ribonuclease inhibitor
From Proteopedia
| Line 4: | Line 4: | ||
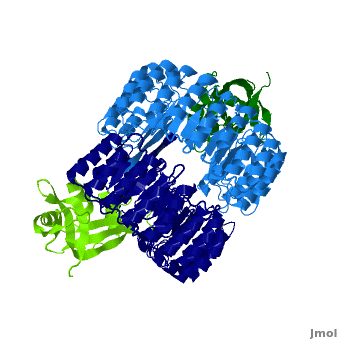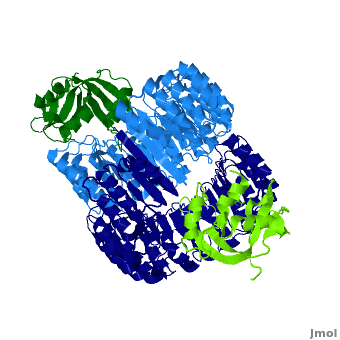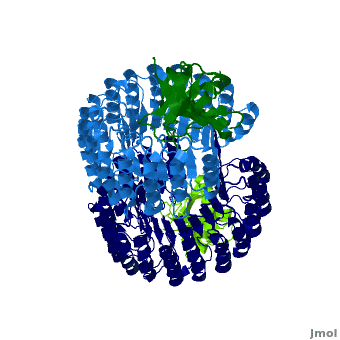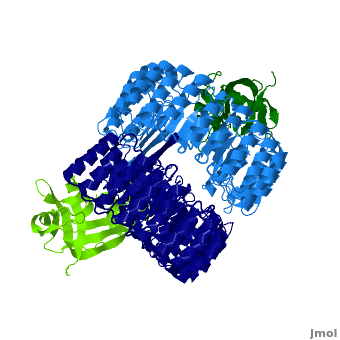
| - | <structuresection load='1z7x' size='550' frame='true' align='right' caption='hRI(blue) complexed with RNase 1(green)' scene='Ribonuclease_inhibitor/1z7x/3' > | + | <structuresection load='1z7x' size='550' frame='true' align='right' caption='hRI(blue) complexed with RNase 1(green), [[1z7x]]' scene='Ribonuclease_inhibitor/1z7x/3' > |
==Structural Motifs== | ==Structural Motifs== | ||
RI features 15 leucine-rich-repeats (LRR) alternating 28 and 29 residues. <scene name='Ribonuclease_inhibitor/Motiffs/4'>Alpha helices</scene> make up the outer circumference with <scene name='Ribonuclease_inhibitor/Motiffs/2'>parallel beta sheets</scene> on the inner circumference. There are no disulfide bonds in the <scene name='Ribonuclease_inhibitor/Motiffs/3'>horseshoe shaped structure.</scene> While RI undergoes a conformation change upon binding of a substrate, there is no hinge. The lack of long-range stabilization allows for structural flexibility especially between the two ends of the molecule. | RI features 15 leucine-rich-repeats (LRR) alternating 28 and 29 residues. <scene name='Ribonuclease_inhibitor/Motiffs/4'>Alpha helices</scene> make up the outer circumference with <scene name='Ribonuclease_inhibitor/Motiffs/2'>parallel beta sheets</scene> on the inner circumference. There are no disulfide bonds in the <scene name='Ribonuclease_inhibitor/Motiffs/3'>horseshoe shaped structure.</scene> While RI undergoes a conformation change upon binding of a substrate, there is no hinge. The lack of long-range stabilization allows for structural flexibility especially between the two ends of the molecule. | ||
| Line 24: | Line 24: | ||
</structuresection> | </structuresection> | ||
==Medical Implications== | ==Medical Implications== | ||
| - | As mentioned earlier in the introduction, ribonucleases are cytotoxic. They bind to and chop up RNA. RNase is an endonuclease and is diffusion limited, meaning it acts as fast as susbstrates arrive. RNases exhibit great stability and are often purified by sulfuric acid treatment and then boiling until it is the only surviving macromolecule. RI’s exist to protect cells from rogue RNases. <ref>http://www.pdb.org/pdb/101/motm.do?momID=105</ref> <br> <br> As mentioned earlier the amphibian RNase ONC is currently in clinical trials. Human ribonucleases possess advantages over amphibian, namely increased catalytic ability, decreased renal toxicity, and decreased immunogenicity. The RI evasion of an RNase is critical to its chemotherapeutic effectiveness. Genetic engineers have been working on site-specific mutations that either decrease the association constant or increase the disassociation constant. However, altering residues in exchange for RI evasion is a double-edged sword because cytotoxicity must be preserved. One particular species, “R39D/N67D/N88A/ G89D/R91D RNase 1" has a 5×10^9-fold decrease in affinity for RI, while maintaing nearly wild-type ribonucleolytic activity, conformational stability, and cytotoxicity. <ref>PMID:17350650</ref> The R39D and R91D substitutions sever the hydrogen bonds formed by <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> that served as electrostatic targeting regions. The aspartates create negative/negative repulsion. This modified RNase provides an interesting example of the future direction and potential of biochemistry and medicine. | + | As mentioned earlier in the introduction, ribonucleases are cytotoxic. They bind to and chop up RNA. RNase is an endonuclease and is diffusion limited, meaning it acts as fast as susbstrates arrive. RNases exhibit great stability and are often purified by sulfuric acid treatment and then boiling until it is the only surviving macromolecule. RI’s exist to protect cells from rogue RNases. <ref>http://www.pdb.org/pdb/101/motm.do?momID=105</ref> <br> <br> As mentioned earlier the amphibian RNase ONC is currently in clinical trials. Human ribonucleases possess advantages over amphibian, namely increased catalytic ability, decreased renal toxicity, and decreased immunogenicity. The RI evasion of an RNase is critical to its chemotherapeutic effectiveness. Genetic engineers have been working on site-specific mutations that either decrease the association constant or increase the disassociation constant. However, altering residues in exchange for RI evasion is a double-edged sword because cytotoxicity must be preserved. One particular species, “R39D/N67D/N88A/ G89D/R91D RNase 1" has a 5×10^9-fold decrease in affinity for RI, while maintaing nearly wild-type ribonucleolytic activity, conformational stability, and cytotoxicity. <ref>PMID:17350650</ref> The R39D and R91D substitutions sever the hydrogen bonds formed by <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> and <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> that served as electrostatic targeting regions. The aspartates create negative/negative repulsion. This modified RNase provides an interesting example of the future direction and potential of biochemistry and medicine. |
==3d structures== | ==3d structures== | ||
Revision as of 07:15, 9 November 2011
Ribonuclease inhibitors (RI) are a family of large (~450 residues, ~49 kDa), acidic (pI ~4.7), proteins that catalyze the degradation of ribonucleases. Human RI(hRI) is a major cellular protein, comprising ~0.1% of all cellular protein by weight. [1]
Ribonucleases (RNase) are enzymes that degrade RNA and are often cytotoxic which gives them chemotherapeutic properties. However, when bound to an RI they are no longer functional. Understanding the mechanism through which RI identifies and binds to RNases will allow scientists to design/modify RNases to evade hRI. In fact, one drug, Onconase (ONC), a ribonuclease from the Northern Leopard Frog (Rana pipiens), is now in Phase III clinical trials as a cancer chemotherapeutic agent [2].
| |||||||||||
Medical Implications
As mentioned earlier in the introduction, ribonucleases are cytotoxic. They bind to and chop up RNA. RNase is an endonuclease and is diffusion limited, meaning it acts as fast as susbstrates arrive. RNases exhibit great stability and are often purified by sulfuric acid treatment and then boiling until it is the only surviving macromolecule. RI’s exist to protect cells from rogue RNases. [8]
As mentioned earlier the amphibian RNase ONC is currently in clinical trials. Human ribonucleases possess advantages over amphibian, namely increased catalytic ability, decreased renal toxicity, and decreased immunogenicity. The RI evasion of an RNase is critical to its chemotherapeutic effectiveness. Genetic engineers have been working on site-specific mutations that either decrease the association constant or increase the disassociation constant. However, altering residues in exchange for RI evasion is a double-edged sword because cytotoxicity must be preserved. One particular species, “R39D/N67D/N88A/ G89D/R91D RNase 1" has a 5×10^9-fold decrease in affinity for RI, while maintaing nearly wild-type ribonucleolytic activity, conformational stability, and cytotoxicity. [9] The R39D and R91D substitutions sever the hydrogen bonds formed by and that served as electrostatic targeting regions. The aspartates create negative/negative repulsion. This modified RNase provides an interesting example of the future direction and potential of biochemistry and medicine.
3d structures
References
- ↑ Shapiro R. Cytoplasmic ribonuclease inhibitor. Methods Enzymol. 2001;341:611-28. PMID:11582809
- ↑ Zwolinska M, Smolewski P. [Onconase: a ribonuclease with antitumor activity]. Postepy Hig Med Dosw (Online). 2010 Feb 19;64:58-66. PMID:20173221
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
- ↑ Kobe B, Deisenhofer J. Mechanism of ribonuclease inhibition by ribonuclease inhibitor protein based on the crystal structure of its complex with ribonuclease A. J Mol Biol. 1996 Dec 20;264(5):1028-43. PMID:9000628 doi:http://dx.doi.org/10.1006/jmbi.1996.0694
- ↑ Kobe B, Deisenhofer J. Mechanism of ribonuclease inhibition by ribonuclease inhibitor protein based on the crystal structure of its complex with ribonuclease A. J Mol Biol. 1996 Dec 20;264(5):1028-43. PMID:9000628 doi:http://dx.doi.org/10.1006/jmbi.1996.0694
- ↑ http://www.pdb.org/pdb/101/motm.do?momID=105
- ↑ Johnson RJ, McCoy JG, Bingman CA, Phillips GN Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007 Apr 27;368(2):434-49. Epub 2007 Feb 9. PMID:17350650 doi:10.1016/j.jmb.2007.02.005
Proteopedia Page Contributors and Editors (what is this?)
Abe Weintraub, Michal Harel, Alexander Berchansky, Joel L. Sussman