Acetylcholinesterase inhibitors
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='2wg1' size='500' side='right' caption='Structure of AChE with Soman and 2 PAM (PDB entry [[2wg1]])' scene=''> | <StructureSection load='2wg1' size='500' side='right' caption='Structure of AChE with Soman and 2 PAM (PDB entry [[2wg1]])' scene=''> | ||
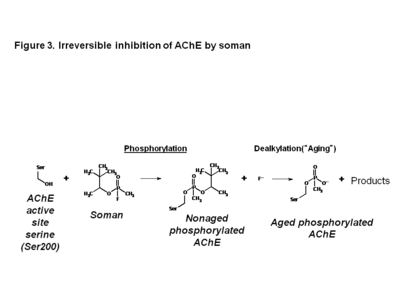
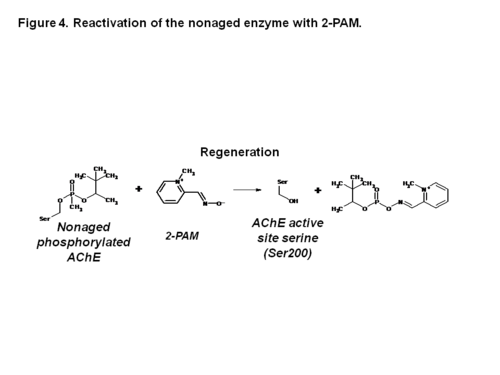
| - | '''Acetylcholinesterase (AChE)''' is an enzyme that catalyses the hydrolysis of the neurotransmitter acetylcholine (ACh) to acetate and choline, and plays a crucial role in terminating the nerve impulse at cholinergic synapses. Reversible inhibitors of AChE are used to treat diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Conversely, irreversible inhibition of AChE by organophosphates (<scene name='Acetylcholinesterase_inhibitors/Phosphorus/3'>OP</scene>), such as soman, can lead to death. In this process, the OP covalently attaches to a <scene name='Acetylcholinesterase_inhibitors/Serine_residue/5'>serine residue</scene> in the active site to generate a <scene name='Acetylcholinesterase_inhibitors/Ser200_and_op/1'>phosphorylated enzyme</scene>. Subsequently, the OP is dealkylated, a process that is termed aging, generating a form of the enzyme that cannot be reactivated by antidotes, such as pralidoxime (2-PAM). Recently, the structures of Torpedo californica (Tc) AChE conjugated with soman, in both the non-aged and aged forms, and 2-PAM have been solved. From these structures, it is clear that a structural reorientation of His440, a component of the active site, occurs during the aging process. Additionally, the structure of the ternary complex of the aged AChE complex with <scene name='Acetylcholinesterase_inhibitors/2_pam/1'>2-PAM</scene>, revealed that the oxime functional group is not optimally positioned for nucleophilic attack on the phosphorous atom.<ref name=”practice”> PMID: 19642642</ref> | + | '''[[Acetylcholinesterase]] (AChE)''' is an enzyme that catalyses the hydrolysis of the neurotransmitter acetylcholine (ACh) to acetate and choline, and plays a crucial role in terminating the nerve impulse at cholinergic synapses. Reversible inhibitors of AChE are used to treat diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Conversely, irreversible inhibition of AChE by organophosphates (<scene name='Acetylcholinesterase_inhibitors/Phosphorus/3'>OP</scene>), such as soman, can lead to death. In this process, the OP covalently attaches to a <scene name='Acetylcholinesterase_inhibitors/Serine_residue/5'>serine residue</scene> in the active site to generate a <scene name='Acetylcholinesterase_inhibitors/Ser200_and_op/1'>phosphorylated enzyme</scene>. Subsequently, the OP is dealkylated, a process that is termed aging, generating a form of the enzyme that cannot be reactivated by antidotes, such as pralidoxime (2-PAM). Recently, the structures of Torpedo californica (Tc) AChE conjugated with soman, in both the non-aged and aged forms, and 2-PAM have been solved. From these structures, it is clear that a structural reorientation of His440, a component of the active site, occurs during the aging process. Additionally, the structure of the ternary complex of the aged AChE complex with <scene name='Acetylcholinesterase_inhibitors/2_pam/1'>2-PAM</scene>, revealed that the oxime functional group is not optimally positioned for nucleophilic attack on the phosphorous atom.<ref name=”practice”> PMID: 19642642</ref> |
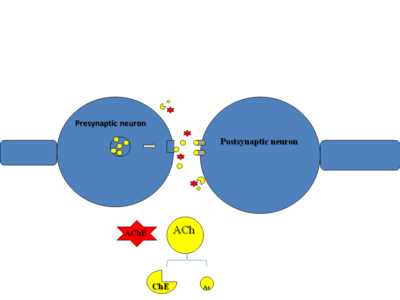
ACh is a neurotransmitter that is essential for nerve transmission at the cholinergic synapse (Figure 1). In the beginning a nerve impulse reaches the presynaptic membrane which acts upon vesicles that contain ACh. Then ACh is released into the synapse. Afterwards, ACh diffuses across the synapse where it binds and activates receptors on the postsynaptic neuron. As a result a nerve impulse is initiated. It is also important to terminate the impulse by breaking down ACh into acetyl and choline portions by AChE. | ACh is a neurotransmitter that is essential for nerve transmission at the cholinergic synapse (Figure 1). In the beginning a nerve impulse reaches the presynaptic membrane which acts upon vesicles that contain ACh. Then ACh is released into the synapse. Afterwards, ACh diffuses across the synapse where it binds and activates receptors on the postsynaptic neuron. As a result a nerve impulse is initiated. It is also important to terminate the impulse by breaking down ACh into acetyl and choline portions by AChE. | ||
[[Image:slide3.png|right|400px|thumb| [[Figure 1]]]] | [[Image:slide3.png|right|400px|thumb| [[Figure 1]]]] | ||
| Line 42: | Line 42: | ||
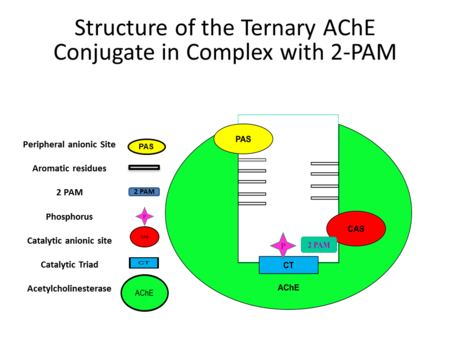
| - | [[Image:slide8.png| | + | [[Image:slide8.png|right|450px|thumb| [[Figure 5]]]] |
| - | Figure 5 shows a detailed image of the interaction among different molecules | + | Figure 5* shows a detailed image of the interaction among different molecules |
| - | and their positions in Acetylcholinesterase. | + | and their respective positions in Acetylcholinesterase. |
| - | + | ||
| Line 62: | Line 61: | ||
Decamethonium and heptylene linked bistacrene which are longer but act in a similar mode to the | Decamethonium and heptylene linked bistacrene which are longer but act in a similar mode to the | ||
Oximes. <ref>PMID: 19642642</ref> | Oximes. <ref>PMID: 19642642</ref> | ||
| - | |||
| - | |||
| - | |||
| Line 92: | Line 88: | ||
reactivated since this agent would not let OP bind to AChE. Currently it seems that this agent is PON1, serum | reactivated since this agent would not let OP bind to AChE. Currently it seems that this agent is PON1, serum | ||
paraoxonase, however more research has to be done in order to see its interaction with all/most OP. | paraoxonase, however more research has to be done in order to see its interaction with all/most OP. | ||
| + | |||
'''Acknowledgements''' | '''Acknowledgements''' | ||
| Line 98: | Line 95: | ||
Professor Lars Westblade, Touro College of Pharmacy | Professor Lars Westblade, Touro College of Pharmacy | ||
| - | |||
| - | Hostos-Lincoln Academy SMART Team 2009-10 and Ms. Allison Granberry | ||
Naomi Arye, Touro College of Pharmacy | Naomi Arye, Touro College of Pharmacy | ||
Yakov Fattakhov, Touro College of Pharmacy | Yakov Fattakhov, Touro College of Pharmacy | ||
| + | |||
| + | Hostos-Lincoln Academy SMART Team 2009-10* | ||
| + | |||
| + | |||
Current revision
| |||||||||||