PcrA helicase
From Proteopedia
(→Structure of Pcr4 DNA Helicase) |
|||
| (67 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image: | + | [[Image:1jprto3jpr.png|left|300px]] |
<!-- | <!-- | ||
| Line 7: | Line 7: | ||
or leave the SCENE parameter empty for the default display. | or leave the SCENE parameter empty for the default display. | ||
--> | --> | ||
| - | + | <!--The line below this paragraph, {{ABSTRACT_PUBMED_8934527}}, adds the Publication Abstract to the page | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <!-- | + | |
| - | The line below this paragraph, {{ABSTRACT_PUBMED_8934527}}, adds the Publication Abstract to the page | + | |
(as it appears on PubMed at http://www.pubmed.gov), where 8934527 is the PubMed ID number. | (as it appears on PubMed at http://www.pubmed.gov), where 8934527 is the PubMed ID number. | ||
--> | --> | ||
| - | {{ABSTRACT_PUBMED_8934527}} | + | <!--{{ABSTRACT_PUBMED_8934527}} --> |
''' | ''' | ||
| - | <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/1pjrglobular/1'>TextToBeDisplayed</scene> | ||
| - | Helicases are nucleic acid–dependent ATP-ases that are capable of unwinding DNA [http://en.wikipedia.org/wiki/DNA] or RNA [http://en.wikipedia.org/wiki/RNA] duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1). | ||
| - | PcrA is part of the helicase superfamily I. A monomeric protein that is mainly alfa helical | ||
| - | <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/Initial/1'>secondary structure</scene> | ||
| - | + | == What is a Helicase? == | |
| - | + | {{STRUCTURE_1pjr| PDB=1pjr | SIZE=400| SCENE= |right|CAPTION=PcrA , [[1pjr]] }} | |
| - | + | <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/1pjrglobular/1'>Spacefill</scene> | |
| - | + | Helicases are nucleic acid–dependent ATP-ases that are capable of unwinding DNA [http://en.wikipedia.org/wiki/DNA] or RNA [http://en.wikipedia.org/wiki/RNA] duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1). | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ==PcrA a Simple Model for Helicases== | ||
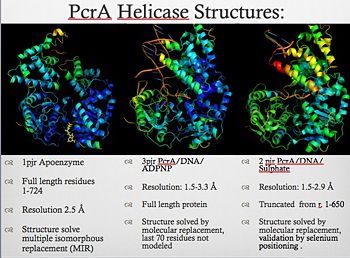
| + | [[Image:Pcr4_structures.jpg|thumb|350px|left|PcrA_Structure]] | ||
| + | PcrA is part of the replication machinery of the [http://en.wikipedia.org/wiki/Geobacillus_stearothermophilus Geobacillus stearothermophilus]a gram (+) bacteria, This helicase is part of the superfamily I of Helicases. Monomeric protein that is mainly <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/Initial/1'>alfa helical</scene> has the <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/1pjrconser/2'>highly conserved</scene> Rec domians. This helicase was reported as a mutation in the gen PcrA from [http://en.wikipedia.org/wiki/staphylococcu "Stapphylococcus aerous"], this mutation was related to a deficiency in the replication of a reporter plasmid.[http://www.ncbi.nlm.nih.gov/pubmed/8232203?ordinalpos=81&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum] | ||
<table align='right'><tr><td> </td><td>{{Template:ColorKey_ConSurf}}</td></tr></table> | <table align='right'><tr><td> </td><td>{{Template:ColorKey_ConSurf}}</td></tr></table> | ||
| - | + | {{clear}} | |
| - | + | ==PcrA Biochemistry== | |
| - | The | + | PcrA is has an ATPas activityt which directionality is from 3' to 5' helicase strand separation reaction. The enzyme shows a specificity for the DNA substrate in gel mobility assays with the preferred substrate being one containing both single and double stranded regions of DNA. In contrast to Rep and UvrD from E. coli, there is not evidence for dimerisation of the enzyme using gel filtration, or by crosslinking in the presence of combinations of Mg2+, nucleotides and DNA. Moreover, kcat for ATP hydrolysis is constant over a large range of protein concentrations. Therefore, the protein appears to be monomeric under all conditions tested, including in the structure of two crystal forms of PcrA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html] |
| + | [[Image:Wigleypcr4.jpg]] | ||
| - | == | + | ==PcrA Helicase Mechanism : The Mexican Wave== |
| - | ====Non-Specific Binding==== | ||
| - | <!-- | ||
| - | <table width='350' align='right' cellpadding='5'><tr><td rowspan='2'> </td><td bgcolor='#d0d0d0'> | ||
| - | --> | ||
| - | <applet load='Image:1osl_ca.pdb' size='450' frame='true' align='right' scene='Lac_repressor/1osl_ca_dot_pdb/2' /> | ||
| - | <!-- | ||
| - | </td></tr><tr><td bgcolor='#d0d0d0'>[[Morphs|Morph]] of the lac repressor bending DNA as binding changes from non-specific ([[1osl]]) to specific recognition of the operator sequence ([[1l1m]]).</td></tr></table> | ||
| - | --> | ||
| - | + | {{STRUCTURE_3pjr| PDB=3pjr | SIZE=400| SCENE= |right|CAPTION=PcrA complex with DNA and ATP, [[3pjr]] }} | |
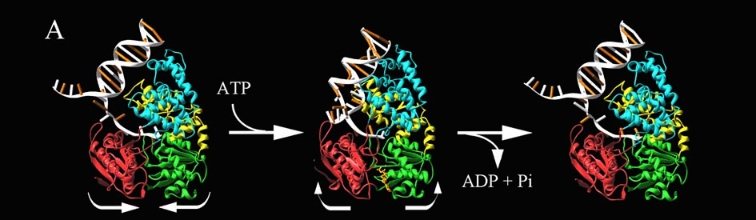
| - | ==== | + | Professor Dale B. Wigley' group in 1996-1999 was able to crystalize the intermediate states from PcrA, giving solution to the controversy of what kind of mechanism this helicase has. [http://www.ncbi.nlm.nih.gov/pubmed/10199404ordinalpos=39&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum] |
| + | Two crystal form of the enzyma, one couple with a 10 mer DNA and a non hydrolizable form of ATP (ATPnP) (pdb id: 3pjr [[3pjr]], <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/3pjrinitial/1'>(Enzyme Subtrate Structure) </scene>and another a truncated form embebed in sulfate (pdb id: 2pjr [[2pjr]]<scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/2pjrinitial/1'>(Enzyme Product Structure)</scene>, give a light in a model for how ATP hydrolysis results in motor movement along ssDNA. In the figure below step 1 (top) is the ATP free (product) ssDNA conformation. The DNA bases are labelled arbitrarily. On binding ATP, F626 creates a new binding pocket for base 6. Likewise, F64 destroys an acceptor pocket for base 2, forcing it to move to the position occupied by base 1. After ATP hydrolysis, the grip on base 6 is released. When the Y257 pocket is re-opened due to movement of F64, bases 3-6 can now flip through the acceptor pockets to their new positions. This model predicts that each ATP hydrolysis event will advance PcrA one base along ssDNA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html] | ||
| + | [[Image:Mexicanwave.jpg|thumb|170px|left|Inchworm or Mexicanwave model]] | ||
| + | [[Image:Snapshot_2008-12-03_14-11-31.jpg|thumb|400px|left|PcrA Movie]] | ||
| - | Upon recognizing the specific operator sequence, the non-specific binding converts to <scene name='Lac_repressor/1l1m_ca_specific_bindiing/3'>specific binding</scene> (derived<ref name='alphac' /> from [[1l1m]], 20 [[NMR Ensembles of Models|NMR models]]). During this conversion, the hinge region changes from disordered loops to {{Template:ColorKey_Helix}} (<scene name='Lac_repressor/1l1m_ca_specific_bindiing/4'>highlight new helices</scene>), which bind in the minor groove of the DNA. This binding opens the minor groove, bending the <font color='#ae00ff'><b>DNA double helix</b></font>. <scene name='Lac_repressor/1l1m_ca_specific_bindiing/6'>Animating</scene> these can be compared with the animation of the non-specific binding. {{Template:Button Toggle Animation}} | ||
| - | ====Morph of Conversion==== | ||
| - | + | '''The link below show a movie with the principal characteristics of this protein as long with the inchworm model'''. [http://www.youtube.com/watch?v=fDwaWCkhgZI Pcr4 Helicase and Mexican Wave] | |
| + | {{Clear}} | ||
| - | The | + | ==The Superfamily 1 (SF1)== |
| + | PcrA share structural domains with the Rec helicases, like UvrD and RepD from E. coli, Superfamily 1 (SF1) helicases are probably the best characterized class, certainly from a structural perspective. All members characterized to date are bona fide helicases and α enzymes. Indeed, from their mode of translocation via the bases it is difficult to envisage how they could translocate along a duplex. However, they can have either A or B directional polarity. | ||
| - | + | {{STRUCTURE_2is1| PDB=2is1 | SIZE=300| SCENE= |left|CAPTION=UVRD complex with DNA and sulfate, [[2is1]] }} | |
| + | {{STRUCTURE_1uaa| PDB=1uaa | SIZE=300| SCENE= |center|CAPTION=REP complex with DNA, [[1uaa]] }} | ||
| - | + | {{clear}} | |
| - | + | ||
| - | + | ||
| - | * In Windows, simply drag the movie and drop it into the Powerpoint slide. You can then resize it and position it. The movie should play when you change the View to Slide Show ("project") the slide. | ||
| - | * In Mac OSX, Ctrl-Click on the movie, then Save Image. In Mac Powerpoint, at the desired slide, use the Insert menu (at the top) and select Movie ..., then insert the saved .gif movie file. After inserting the movie, make sure the Toolbox is showing (controlled with an icon-button at the top of the window). Now you can resize and reposition the movie. Click in the movie in the slide to select it. Now, in the Toolbox/Formatting Palette, under Movie, check Loop Until Stopped. Now the movie should play when you change the View to Slide Show ("project") the slide. | ||
| - | ==Challenge Your Understanding== | ||
| - | Here are some questions to challenge your understanding. | ||
| - | #Why does the lac repressor bind to DNA non-specifically? | ||
| - | #When the lac repressor binds non-specifically to DNA, what part of the DNA double helix does it bind to? | ||
| - | #Does DNA have a net charge, and if so, is it negative or positive in aqueous solution at pH 7? | ||
| - | #What kinds of chemical bonds are likely to be involved in non-specific binding of the repressor protein to DNA? | ||
| - | #Does specific binding of lac repressor to DNA disrupt any of the Watson-Crick hydrogen bonds between the base pairs in the DNA strands? | ||
| - | #How do proteins such as the lac repressor recognize specific nucleotide sequences in a DNA double helix? | ||
| - | #What kinds of chemical bonds are involved in specific binding of the repressor protein to DNA? | ||
| - | #Does the lac repressor recognize specific bases in the major or minor grooves of the DNA? | ||
| - | #Why does the lac repressor bend the DNA double helix when it recognizes its specific nucleotide sequence? | ||
| - | + | ==About this Structure== | |
| + | 1PJR is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Geobacillus_stearothermophilus Geobacillus stearothermophilus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PJR OCA]. | ||
| - | == | + | ==3D structures of helicase== |
| - | + | [[Helicase]] | |
| - | == | + | ==Additional Resources== |
| - | + | For additional information, see: [[DNA Replication, Repair, and Recombination]] | |
| - | + | <br /> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Reference== | ==Reference== | ||
| Line 128: | Line 82: | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Jul 28 01:38:49 2008'' | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Jul 28 01:38:49 2008'' | ||
| + | |||
| + | ^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719. | ||
| + | ^ a b "Researchers solve mystery of how DNA strands separate" (2007-07-03). Retrieved on 2007-07-05. | ||
| + | ^ Dumont S, Cheng W, Serebrov V, Beran RK, Tinoco Jr I, Pylr AM, Bustamante C, "RNA Translocation and Unwinding Mechanism of HCV NS3 Helicase and its Coordination by ATP", Nature. 2006 Jan 5; 439: 105-108. | ||
| + | Anand SP, Zheng H, Bianco PR, Leuba SH, Khan SA. DNA helicase activity of PcrA is not required for displacement of RecA protein from DNA or inhibition of RecA-mediated DNA strand exchange. Journal of Bacteriology (2007) 189 (12):4502-4509. | ||
| + | Bird L, Subramanya HS, Wigley DB, "Helicases: a unifying structural theme?", Current Opinion in Structural Biology. 1998 Feb; 8 (1): 14-18. | ||
| + | Betterton MD, Julicher F, "Opening of nucleic-acid double strands by helicases: active versus passive opening.", Physical Review E. 2005 Jan; 71 (1): 011904. | ||
Current revision
Contents |
What is a Helicase?
| |||||||||
| PcrA , 1pjr | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene: | PCRA (Geobacillus stearothermophilus) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Helicases are nucleic acid–dependent ATP-ases that are capable of unwinding DNA [1] or RNA [2] duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1).
PcrA a Simple Model for Helicases
PcrA is part of the replication machinery of the Geobacillus stearothermophilusa gram (+) bacteria, This helicase is part of the superfamily I of Helicases. Monomeric protein that is mainly has the Rec domians. This helicase was reported as a mutation in the gen PcrA from "Stapphylococcus aerous", this mutation was related to a deficiency in the replication of a reporter plasmid.[3]
 |
PcrA Biochemistry
PcrA is has an ATPas activityt which directionality is from 3' to 5' helicase strand separation reaction. The enzyme shows a specificity for the DNA substrate in gel mobility assays with the preferred substrate being one containing both single and double stranded regions of DNA. In contrast to Rep and UvrD from E. coli, there is not evidence for dimerisation of the enzyme using gel filtration, or by crosslinking in the presence of combinations of Mg2+, nucleotides and DNA. Moreover, kcat for ATP hydrolysis is constant over a large range of protein concentrations. Therefore, the protein appears to be monomeric under all conditions tested, including in the structure of two crystal forms of PcrA.[4]
PcrA Helicase Mechanism : The Mexican Wave
| |||||||||
| PcrA complex with DNA and ATP, 3pjr | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Professor Dale B. Wigley' group in 1996-1999 was able to crystalize the intermediate states from PcrA, giving solution to the controversy of what kind of mechanism this helicase has. [5]
Two crystal form of the enzyma, one couple with a 10 mer DNA and a non hydrolizable form of ATP (ATPnP) (pdb id: 3pjr 3pjr, and another a truncated form embebed in sulfate (pdb id: 2pjr 2pjr, give a light in a model for how ATP hydrolysis results in motor movement along ssDNA. In the figure below step 1 (top) is the ATP free (product) ssDNA conformation. The DNA bases are labelled arbitrarily. On binding ATP, F626 creates a new binding pocket for base 6. Likewise, F64 destroys an acceptor pocket for base 2, forcing it to move to the position occupied by base 1. After ATP hydrolysis, the grip on base 6 is released. When the Y257 pocket is re-opened due to movement of F64, bases 3-6 can now flip through the acceptor pockets to their new positions. This model predicts that each ATP hydrolysis event will advance PcrA one base along ssDNA.[6]
The link below show a movie with the principal characteristics of this protein as long with the inchworm model. Pcr4 Helicase and Mexican Wave
The Superfamily 1 (SF1)
PcrA share structural domains with the Rec helicases, like UvrD and RepD from E. coli, Superfamily 1 (SF1) helicases are probably the best characterized class, certainly from a structural perspective. All members characterized to date are bona fide helicases and α enzymes. Indeed, from their mode of translocation via the bases it is difficult to envisage how they could translocate along a duplex. However, they can have either A or B directional polarity.
| |||||||||
| UVRD complex with DNA and sulfate, 2is1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | uvrD (Escherichia coli) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
| |||||||||
| REP complex with DNA, 1uaa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
About this Structure
1PJR is a Single protein structure of sequence from Geobacillus stearothermophilus. Full crystallographic information is available from OCA.
3D structures of helicase
Additional Resources
For additional information, see: DNA Replication, Repair, and Recombination
Reference
Crystal structure of a DExx box DNA helicase., Subramanya HS, Bird LE, Brannigan JA, Wigley DB, Nature. 1996 Nov 28;384(6607):379-83. PMID:8934527
Page seeded by OCA on Mon Jul 28 01:38:49 2008
^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719. ^ a b "Researchers solve mystery of how DNA strands separate" (2007-07-03). Retrieved on 2007-07-05. ^ Dumont S, Cheng W, Serebrov V, Beran RK, Tinoco Jr I, Pylr AM, Bustamante C, "RNA Translocation and Unwinding Mechanism of HCV NS3 Helicase and its Coordination by ATP", Nature. 2006 Jan 5; 439: 105-108. Anand SP, Zheng H, Bianco PR, Leuba SH, Khan SA. DNA helicase activity of PcrA is not required for displacement of RecA protein from DNA or inhibition of RecA-mediated DNA strand exchange. Journal of Bacteriology (2007) 189 (12):4502-4509. Bird L, Subramanya HS, Wigley DB, "Helicases: a unifying structural theme?", Current Opinion in Structural Biology. 1998 Feb; 8 (1): 14-18. Betterton MD, Julicher F, "Opening of nucleic-acid double strands by helicases: active versus passive opening.", Physical Review E. 2005 Jan; 71 (1): 011904.
Proteopedia Page Contributors and Editors (what is this?)
Luis E Ramirez-Tapia, Michal Harel, Eran Hodis, David Canner