Transthyretin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
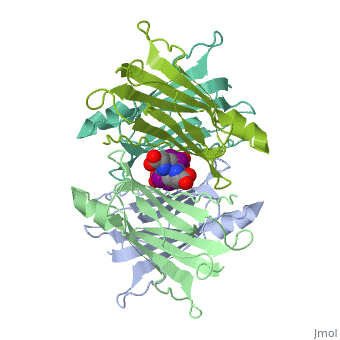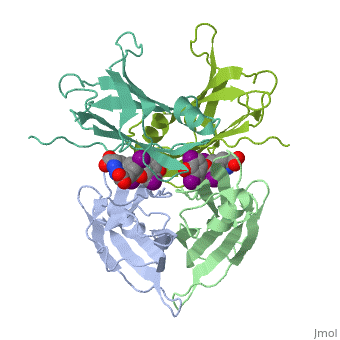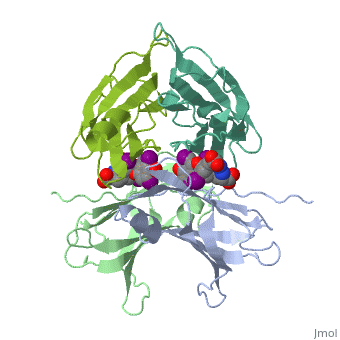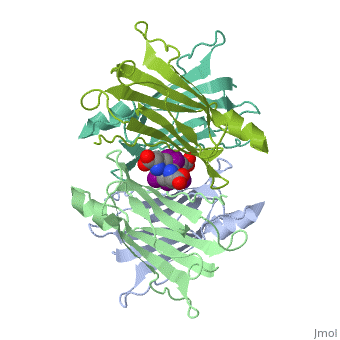
| - | + | <StructureSection load='1tha' size='340' side='right' caption='Human transthyretin complex with tyroxine derivative [[1tha]]' scene=''> | |
| - | <StructureSection load='1tha' size='340' side='right' caption='Human transthyretin | + | |
== Function == | == Function == | ||
'''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. For details see [[Student Project 2 for UMass Chemistry 423 Spring 2015]]. | '''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. For details see [[Student Project 2 for UMass Chemistry 423 Spring 2015]]. | ||
Revision as of 08:00, 22 September 2016
| |||||||||||
3D structures of transthyretin
Updated on 22-September-2016
References
- ↑ Robbins J. Transthyretin from discovery to now. Clin Chem Lab Med. 2002 Dec;40(12):1183-90. PMID:12553418 doi:http://dx.doi.org/10.1515/CCLM.2002.208
- ↑ Saraiva MJ. Transthyretin mutations in health and disease. Hum Mutat. 1995;5(3):191-6. PMID:7599630 doi:http://dx.doi.org/10.1002/humu.1380050302
- ↑ Wojtczak A, Luft J, Cody V. Mechanism of molecular recognition. Structural aspects of 3,3'-diiodo-L-thyronine binding to human serum transthyretin. J Biol Chem. 1992 Jan 5;267(1):353-7. PMID:1730601