User:Scott H. Vanson/Sandbox 1
From Proteopedia
< User:Scott H. Vanson(Difference between revisions)
| (24 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==DNA Polymerase θ== | ==DNA Polymerase θ== | ||
<StructureSection load='4x0p' size='340' side='right' caption='Polymerase θ polymerase domain bound to DNA''> | <StructureSection load='4x0p' size='340' side='right' caption='Polymerase θ polymerase domain bound to DNA''> | ||
| - | + | use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> article describing Jmol <ref>PMID:21638687</ref> | |
| - | + | ||
== General Description == | == General Description == | ||
| - | Human polymerase θ (pol θ) is large, 290kD enzyme consisting of three distinct domains <ref>PMID:23219161</ref><ref name="Seki">PMID:14576298</ref>. An N-terminal helicase-like domain, whose exact cellular functions are a topic of on-going debate and research<ref>PMID:29058711</ref><ref>PMID:29444826</ref>, is linked to a C-terminal, family A DNA polymerase domain by a large and disordered central region<ref name="Seki" />. Notably, pol θ is the only known human polymerase to contain a polymerase and helicase domain in one molecule<ref name="Zahn">PMID: | + | Human polymerase θ (pol θ) is large, 290kD enzyme consisting of three distinct domains <ref>PMID:23219161</ref><ref name="Seki">PMID:14576298</ref>. An N-terminal helicase-like domain, whose exact cellular functions are a topic of on-going debate and research<ref>PMID:29058711</ref><ref>PMID:29444826</ref>, is linked to a C-terminal, family A DNA polymerase domain by a large and disordered central region<ref name="Seki" />. Notably, pol θ is the only known human polymerase to contain a polymerase and helicase domain in one molecule<ref name="Zahn">PMID:25775267</ref>. Crystal structures have been solved for the apo form of the [http://www.rcsb.org/structure/5a9j helicase-like domain] and the ternary complex of the [http://http://www.rcsb.org/structure/4X0P polymerase domain]. The focus of this wiki is the polymerase domain. |
| + | [[Image:2D_PolQ_scheme.png|thumb|left|upright=4]] | ||
| - | + | ===Cellular Functions=== | |
| - | + | Pol θ is thought to promote overall genomic stability by performing several distinct cellular functions. The primary role of the enzyme is to repair of double-stranded DNA breaks as the key enzyme in an error-prone, non-homologous end-joining pathway called alternative end-joining<ref name="Instability">PMID:25275444</ref> or theta-mediated end-joining<ref>PMID:27264557</ref>. Other functions include translesion synthesis, the ability of the polymerase to bypass and extend past a site of oxidative DNA damage<ref>PMID:24648516</ref>, base excision repair <ref>PMID:21917855</ref>, and possibly DNA replication timing <ref>PMID:24989122</ref>. Pol θ has the specialized ability to extend DNA from minimally-paired primers<ref>PMID:17920341</ref>. Repair by this enzyme is considered to error-prone due to its tendency to add or delete short indels <ref name="Instability" />. | |
| + | ===Disease=== | ||
| + | Several types of cancer, such as breast, ovarian, and oral carcinomas, have shown significantly higher expression levels of pol θ and correlate to poorer patient outcomes<ref name="Ceccaldi">PMID:25642963</ref><ref>PMID:20624954</ref><ref>PMID:22987617</ref>. Genomic studies have shown that more than half of epithelial ovarian cancers have defects in the error-free repair pathway of homologous recombination<ref>PMID:21720365</ref> and, as a result, have an increased dependence on theta-mediated end-joining <ref name="Ceccaldi" />. Double-stranded break repair by pol θ may be thought of as a "backup" pathway which cells depend on more when the machinery involved in homologous recombination is compromised or otherwise unavailable. This enzyme has been identified as a potential therapeutic target due to overexpression in cancers in combination with studies that have shown inhibition of pol θ to sensitize human and mouse cells to radiation and chemical agents which induce double-stranded breaks<ref name="Ceccaldi" /><ref>PMID:19630521</ref><ref>PMID:20233878</ref>. | ||
== Structural Highlights == | == Structural Highlights == | ||
| + | Two crystal structures of the polymerase domain have been solved bound to DNA to a resolution of 3.9Å; inserting ddATP opposite tetrahydrofuran (THF, representing an abasic site) with 4 molecules in the asymmetric unit and inserting ddGTP opposite dCMP with 2 molecules in the asymmetric unit<ref name="Zahn" />. An overall assessment of the structures display the canonical <scene name='78/786633/Subdomains/2'>exonuclease, thumb, palm, and fingers subdomains</scene>. The DNA is thought to "sit" on the palm and is enclosed by the thumb and fingers via the minor groove. The palm houses the active site and the fingers move from an open, unbound position to a closed position when bound to DNA, promoting synthesis. The closed conformation of the polymerase domain becomes obvious when compared to the <scene name='78/786633/Taq_pol_i_open/1'>open conformation of a homologous structure</scene>, such as [http://www.rcsb.org/structure/1KTQ Thermus aquaticus DNA polymerase I]. | ||
| + | |||
| + | ===ddATP Opposite THF=== | ||
| + | |||
| + | An inspection of the active site reveals the <scene name='78/786633/Abasic_active_site/1'>conserved residues of the palm subdomain which coordinate the divalent cation</scene>, in this instance calcium. This structure required the use of calcium, a known inhibitor of polymerase activity, as the primer strand retains the 3' hydroxyl which would otherwise be subjected to nucleophilic attack. The similarly conserved <scene name='78/786633/Ddatp_o-helix/2'>lysine and arginine of the O-helix make stabilizing ionic contacts with non-bridging oxygens of the triphosphate tail of the incoming nucleotide</scene>. | ||
| + | |||
| + | ===dCMP Opposite ddGTP=== | ||
| + | |||
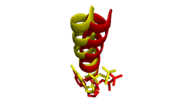
| + | <scene name='78/786633/Mg_complex_overview/1'>This related structure</scene> from the same study was solved with magnesium as the coordinated metal, a 27 amino acid N-terminal truncation, and a blunted DNA oligomer to remove the 3' template overhang. The overall structure is virtually identical to the aforementioned structure complexed with calcium, with the exception of a slight shift in position of the O-helix to be closer to the cognate C:G basepair.[[Image:O-helix_alignment.png|thumb|left|baseline|Alignment of O-helices and incoming nucleotides. Mg2+ complex (yellow) shifts slightly closer to incoming ddGTP than Ca2+ complex (red) relative to its incoming ddATP.]] | ||
== Structural Insights into Function == | == Structural Insights into Function == | ||
| - | == Related Proteins == | + | === Proofreading Activity === |
| + | Family A DNA polymerases harbor an N-terminal exonuclease domain and generally conserve the overall fold. Some members of this family, e.g. [http://www.rcsb.org/structure/1KFD ''E. coli'' pol I], display active 3'-5' proofreading activity via <scene name='78/786633/Pol_i_exonuclease/1'>three catalytic, metal coordinating residues</scene>. Other members, such as pol θ, do not harbor proofreading activity<ref name="Zahn" /> and these three coordinating residues are notably absent at structurally equivalent positions (see below). | ||
| + | [[Image:Pol I exo and pol theta exo.png|thumb|upright=4|Exonuclease domains of active pol I (left) and inactive pol θ (right). Three coordinating residues and metal are absent in pol θ.]] | ||
| + | |||
| + | |||
| + | ===Translesion Synthesis=== | ||
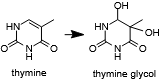
| + | An R2254V variant was made to investigate the importance of this residue in pol θ's ability to extend single-stranded DNA and bypass abasic sites and bulky thymine glycol lesions.[[Image:Thymine_glycol.png|thumb|left|Thymine may oxidized to form a lesion that must be repaired or bypassed.]] Bacterial family A polymerases which do not have this ability retain a valine or leucine at the equivalent position. The R2254V mutant retained its ability to extend double-stranded but not single-stranded DNA and also was not able to bypass abasic sites or thymine glycol. These findings indicated to the authors that <scene name='78/786633/R2254/1'>the salt bridge between R2254 and the 3'-terminal phosphate of the primer</scene> is required to compensate for the missing contacts to the template strand due to a lesion<ref name="Zahn" />. | ||
| + | |||
| + | ====Sticky Thumb==== | ||
| + | In addition to R2254, additional upstream contacts to the primer DNA strand mediated by <scene name='78/786633/Lys_2181/1'>K2181</scene> and <scene name='78/786633/Arg2202/1'>R2202</scene> of the thumb subdomain can be observed. These three salt bridges provide specialized contacts that are not observed in other family A polymerases, in addition to R2201 and R2315 which are also present in [http://www.rcsb.org/structure/4XVK pol ν] and pol I. Alanine substitutions of K2181, R2202, and R2254 resulted in inhibition of pol θ's ability to bypass an abasic site or thymine glycol. These findings prompted the authors to assert that this <scene name='78/786633/Sticky_thumb/1'>specialized thumb domain</scene> may be responsible for the heightened ability of pol θ to synthesize across lesions and to extend from minimally-paired primers<ref name="Zahn" />. | ||
| + | |||
| + | ==Evolutionarily Related Proteins== | ||
| + | DNA polymerases are surprisingly diverse structurally when compared to other enzymes<ref name="Steitz">DOI:10.1074/jbc.274.25.17395</ref>.Polymerase θ is a family A (aka pol I) DNA polymerase, based on shared sequence and structural characteristics. The structure of the palm domain and the ability to coordinate 2 divalent metal ions to catalyze nucleotidyl transfer is shared among all DNA polymerases, with the exception of the pol β family<ref name="Steitz" /><ref>DOI:10.1016/S0959-440X(98)80010-9</ref>. The major differences are located in the thumb, fingers, and exonuclease subdomains. Some examples include that Family A polymerases have additional, conserved interactions with the DNA backbone in the thumb domain and also retain the relative location of the exonuclease subdomain<ref name="Steitz" />. It is important to note that major differences can exist even within a single family, such as an active or inactive exonuclease subdomain, as discussed previously in this article. | ||
| + | |||
| + | Links to other family A DNA polymerases: | ||
| + | |||
| + | [http://www.rcsb.org/structure/1SKR T7 DNA polymerase] | ||
| + | |||
| + | [http://www.rcsb.org/structure/3BDP ''Geobacillus stearothermophilus'' DNA polymerase I] | ||
| + | |||
| + | [http://www.rcsb.org/structure/2KZM ''E. coli'' polymerase I] | ||
| + | |||
| + | [http://www.rcsb.org/structure/1TAQ ''Thermus aquaticus'' DNA polymerase] | ||
| + | |||
| + | [http://www.rcsb.org/structure/2G4C Human polymerase γ (subunit 2)] | ||
| + | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<references/> | <references/> | ||
Current revision
DNA Polymerase θ
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Yousefzadeh MJ, Wood RD. DNA polymerase POLQ and cellular defense against DNA damage. DNA Repair (Amst). 2013 Jan 1;12(1):1-9. doi: 10.1016/j.dnarep.2012.10.004. Epub , 2012 Dec 4. PMID:23219161 doi:http://dx.doi.org/10.1016/j.dnarep.2012.10.004
- ↑ 4.0 4.1 Seki M, Marini F, Wood RD. POLQ (Pol theta), a DNA polymerase and DNA-dependent ATPase in human cells. Nucleic Acids Res. 2003 Nov 1;31(21):6117-26. PMID:14576298
- ↑ Mateos-Gomez PA, Kent T, Deng SK, McDevitt S, Kashkina E, Hoang TM, Pomerantz RT, Sfeir A. The helicase domain of Poltheta counteracts RPA to promote alt-NHEJ. Nat Struct Mol Biol. 2017 Dec;24(12):1116-1123. doi: 10.1038/nsmb.3494. Epub 2017, Oct 23. PMID:29058711 doi:http://dx.doi.org/10.1038/nsmb.3494
- ↑ Ozdemir AY, Rusanov T, Kent T, Siddique LA, Pomerantz RT. Polymerase theta-helicase efficiently unwinds DNA and RNA-DNA hybrids. J Biol Chem. 2018 Apr 6;293(14):5259-5269. doi: 10.1074/jbc.RA117.000565. Epub, 2018 Feb 14. PMID:29444826 doi:http://dx.doi.org/10.1074/jbc.RA117.000565
- ↑ 7.0 7.1 7.2 7.3 7.4 Zahn KE, Averill AM, Aller P, Wood RD, Doublie S. Human DNA polymerase theta grasps the primer terminus to mediate DNA repair. Nat Struct Mol Biol. 2015 Mar 16. doi: 10.1038/nsmb.2993. PMID:25775267 doi:http://dx.doi.org/10.1038/nsmb.2993
- ↑ 8.0 8.1 Yousefzadeh MJ, Wyatt DW, Takata K, Mu Y, Hensley SC, Tomida J, Bylund GO, Doublie S, Johansson E, Ramsden DA, McBride KM, Wood RD. Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS Genet. 2014 Oct 2;10(10):e1004654. doi: 10.1371/journal.pgen.1004654., eCollection 2014 Oct. PMID:25275444 doi:http://dx.doi.org/10.1371/journal.pgen.1004654
- ↑ Wood RD, Doublie S. DNA polymerase theta (POLQ), double-strand break repair, and cancer. DNA Repair (Amst). 2016 Aug;44:22-32. doi: 10.1016/j.dnarep.2016.05.003. Epub, 2016 May 14. PMID:27264557 doi:http://dx.doi.org/10.1016/j.dnarep.2016.05.003
- ↑ Yoon JH, Roy Choudhury J, Park J, Prakash S, Prakash L. A role for DNA polymerase theta in promoting replication through oxidative DNA lesion, thymine glycol, in human cells. J Biol Chem. 2014 May 9;289(19):13177-85. doi: 10.1074/jbc.M114.556977. Epub 2014, Mar 19. PMID:24648516 doi:http://dx.doi.org/10.1074/jbc.M114.556977
- ↑ Asagoshi K, Lehmann W, Braithwaite EK, Santana-Santos L, Prasad R, Freedman JH, Van Houten B, Wilson SH. Single-nucleotide base excision repair DNA polymerase activity in C. elegans in the absence of DNA polymerase beta. Nucleic Acids Res. 2012 Jan;40(2):670-81. doi: 10.1093/nar/gkr727. Epub 2011 Sep , 14. PMID:21917855 doi:http://dx.doi.org/10.1093/nar/gkr727
- ↑ Fernandez-Vidal A, Guitton-Sert L, Cadoret JC, Drac M, Schwob E, Baldacci G, Cazaux C, Hoffmann JS. A role for DNA polymerase theta in the timing of DNA replication. Nat Commun. 2014 Jul 3;5:4285. doi: 10.1038/ncomms5285. PMID:24989122 doi:http://dx.doi.org/10.1038/ncomms5285
- ↑ Seki M, Wood RD. DNA polymerase theta (POLQ) can extend from mismatches and from bases opposite a (6-4) photoproduct. DNA Repair (Amst). 2008 Jan 1;7(1):119-27. doi: 10.1016/j.dnarep.2007.08.005., Epub 2007 Oct 24. PMID:17920341 doi:http://dx.doi.org/10.1016/j.dnarep.2007.08.005
- ↑ 14.0 14.1 14.2 Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI, O'Connor KW, Konstantinopoulos PA, Elledge SJ, Boulton SJ, Yusufzai T, D'Andrea AD. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature. 2015 Feb 12;518(7538):258-62. doi: 10.1038/nature14184. Epub 2015 Feb 2. PMID:25642963 doi:http://dx.doi.org/10.1038/nature14184
- ↑ Lemee F, Bergoglio V, Fernandez-Vidal A, Machado-Silva A, Pillaire MJ, Bieth A, Gentil C, Baker L, Martin AL, Leduc C, Lam E, Magdeleine E, Filleron T, Oumouhou N, Kaina B, Seki M, Grimal F, Lacroix-Triki M, Thompson A, Roche H, Bourdon JC, Wood RD, Hoffmann JS, Cazaux C. DNA polymerase theta up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13390-5. doi:, 10.1073/pnas.0910759107. Epub 2010 Jul 12. PMID:20624954 doi:http://dx.doi.org/10.1073/pnas.0910759107
- ↑ Lessa RC, Campos AH, Freitas CE, Silva FR, Kowalski LP, Carvalho AL, Vettore AL. Identification of upregulated genes in oral squamous cell carcinomas. Head Neck. 2013 Oct;35(10):1475-81. doi: 10.1002/hed.23169. Epub 2012 Sep 18. PMID:22987617 doi:http://dx.doi.org/10.1002/hed.23169
- ↑ . Integrated genomic analyses of ovarian carcinoma. Nature. 2011 Jun 29;474(7353):609-15. doi: 10.1038/nature10166. PMID:21720365 doi:http://dx.doi.org/10.1038/nature10166
- ↑ Goff JP, Shields DS, Seki M, Choi S, Epperly MW, Dixon T, Wang H, Bakkenist CJ, Dertinger SD, Torous DK, Wittschieben J, Wood RD, Greenberger JS. Lack of DNA polymerase theta (POLQ) radiosensitizes bone marrow stromal cells in vitro and increases reticulocyte micronuclei after total-body irradiation. Radiat Res. 2009 Aug;172(2):165-74. doi: 10.1667/RR1598.1. PMID:19630521 doi:http://dx.doi.org/10.1667/RR1598.1
- ↑ Higgins GS, Prevo R, Lee YF, Helleday T, Muschel RJ, Taylor S, Yoshimura M, Hickson ID, Bernhard EJ, McKenna WG. A small interfering RNA screen of genes involved in DNA repair identifies tumor-specific radiosensitization by POLQ knockdown. Cancer Res. 2010 Apr 1;70(7):2984-93. doi: 10.1158/0008-5472.CAN-09-4040. Epub, 2010 Mar 16. PMID:20233878 doi:http://dx.doi.org/10.1158/0008-5472.CAN-09-4040
- ↑ 20.0 20.1 20.2 Steitz TA. DNA polymerases: structural diversity and common mechanisms. J Biol Chem. 1999 Jun 18;274(25):17395-8. PMID:10364165
- ↑ Brautigam CA, Steitz TA. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr Opin Struct Biol. 1998 Feb;8(1):54-63. doi: 10.1016/s0959-440x(98)80010-9. PMID:9519297 doi:http://dx.doi.org/10.1016/s0959-440x(98)80010-9