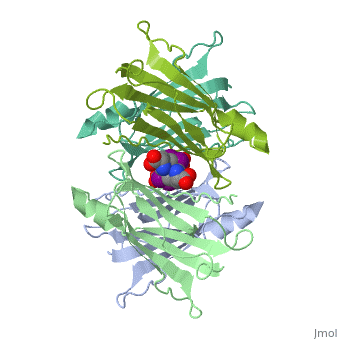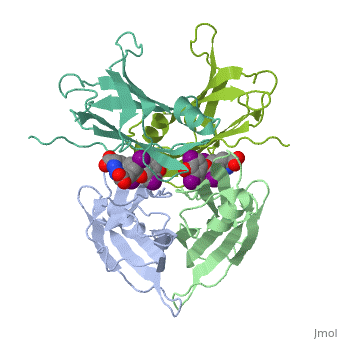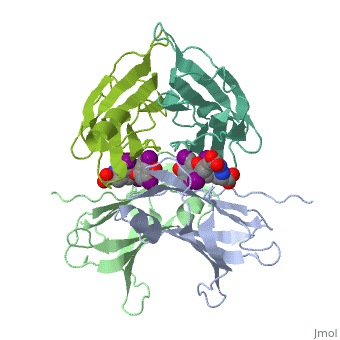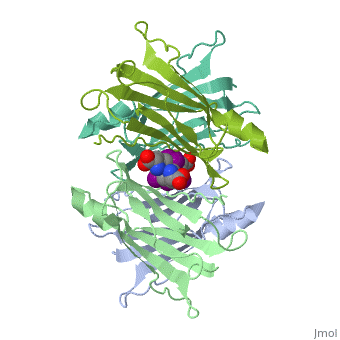
Transthyretin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='1tha' size=' | + | <StructureSection load='1tha' size='350' side='right' caption='Human transthyretin complex with tyroxine derivative [[1tha]]' scene='' pspeed='8'> |
== Function == | == Function == | ||
'''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. For details see [[Student Project 2 for UMass Chemistry 423 Spring 2015]]. | '''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. For details see [[Student Project 2 for UMass Chemistry 423 Spring 2015]]. | ||
| Line 46: | Line 46: | ||
**[[4abq]], [[4abv]], [[4abw]], [[4ac2]], [[4ac4]], [[4act]], [[4ank]], [[4abu]] – hTTR + ligand<br /> | **[[4abq]], [[4abv]], [[4abw]], [[4ac2]], [[4ac4]], [[4act]], [[4ank]], [[4abu]] – hTTR + ligand<br /> | ||
**[[4des]], [[4det]], [[4der]], [[4dew]], [[4deu]] – hTTR + flavonoid<br /> | **[[4des]], [[4det]], [[4der]], [[4dew]], [[4deu]] – hTTR + flavonoid<br /> | ||
| - | **[[1u21]], [[3cfn]], [[3cfq]], [[3cft]], [[3hj0]], [[3nee]], [[3neo]], [[3p3r]], [[3p3s]], [[3p3t]], [[3p3u]], [[4ky2]], [[4mas]], [[5e23]], [[5e4a]], [[5e4o]], [[5ezp]], [[5ayt]], [[4ydm]], [[4ydn]], [[4tq8]], [[4tqh]], [[4tqi]], [[4tqp]], [[5jim]], [[5jid]] – hTTR + inhibitor | + | **[[1u21]], [[3cfn]], [[3cfq]], [[3cft]], [[3hj0]], [[3nee]], [[3neo]], [[3p3r]], [[3p3s]], [[3p3t]], [[3p3u]], [[4ky2]], [[4mas]], [[5e23]], [[5e4a]], [[5e4o]], [[5ezp]], [[5ayt]], [[4ydm]], [[4ydn]], [[4tq8]], [[4tqh]], [[4tqi]], [[4tqp]], [[5jim]], [[5jid]] – hTTR + inhibitor<br /> |
| + | **[[3nes]], [[3nex]] – hTTR (mutant) + inhibitor<br /> | ||
**[[1e3f]], [[1e4h]], [[1e5a]], [[2b15]], [[5hjg]], [[4l4j]] – hTTR + phenol derivative<br /> | **[[1e3f]], [[1e4h]], [[1e5a]], [[2b15]], [[5hjg]], [[4l4j]] – hTTR + phenol derivative<br /> | ||
**[[4l1s]] - hTTR + benzamide derivative<br /> | **[[4l1s]] - hTTR + benzamide derivative<br /> | ||
**[[4l1t]] - hTTR + benzoate derivative<br /> | **[[4l1t]] - hTTR + benzoate derivative<br /> | ||
**[[5jiq]] - hTTR + benzophenone derivative<br /> | **[[5jiq]] - hTTR + benzophenone derivative<br /> | ||
| - | **[[5l4m]] - hTTR + pyridine derivative<br /> | + | **[[5l4m]], [[6grp]] - hTTR + pyridine derivative<br /> |
**[[4n85]], [[4n86]] - hTTR + glabridin<br /> | **[[4n85]], [[4n86]] - hTTR + glabridin<br /> | ||
**[[4n87]] - hTTR (mutant) + glabridin<br /> | **[[4n87]] - hTTR (mutant) + glabridin<br /> | ||
| Line 59: | Line 60: | ||
**[[3ng5]] – hTTR (mutant) + epigallocatechin gallate | **[[3ng5]] – hTTR (mutant) + epigallocatechin gallate | ||
| - | |||
| - | **[[3nes]], [[3nex]] – hTTR (mutant) + inhibitor | ||
**[[1tyr]] – hTTR + retinoic acid | **[[1tyr]] – hTTR + retinoic acid | ||
| Line 94: | Line 93: | ||
**[[4wo0]] - hTTR + apigenin<br /> | **[[4wo0]] - hTTR + apigenin<br /> | ||
**[[5boj]] - hTTR + gemfibrozil<br /> | **[[5boj]] - hTTR + gemfibrozil<br /> | ||
| + | **[[6gnm]] - sbTTR + benzophenone derivative - sea bream<br /> | ||
| + | **[[6gno]], [[6gnw]] - sbTTR + phenol derivative <br /> | ||
| + | **[[6gnp]], [[6gnr]] - sbTTR + pyridine derivative <br /> | ||
| + | **[[6gon]], [[6goo]] - sbTTR + perfluorooctanoate <br /> | ||
| - | * | + | *Transthyretin ternary complexes |
**[[4pme]] - hTTR + ferulic acid + curcumin<br /> | **[[4pme]] - hTTR + ferulic acid + curcumin<br /> | ||
| Line 121: | Line 124: | ||
**[[1sn5]] – gsTTR + T4 derivative | **[[1sn5]] – gsTTR + T4 derivative | ||
| + | |||
| + | *Transthyretin peptide amyloid forming | ||
| + | |||
| + | **[[6c3f]], [[6c3g]], [[6c3s]], [[6c3t]], [[6c4o]], [[6c88]], [[4xfn]], [[4xfo]] - hTTR <br /> | ||
}} | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 16:51, 27 September 2018
| |||||||||||
3D structures of transthyretin
Updated on 27-September-2018
References
- ↑ Robbins J. Transthyretin from discovery to now. Clin Chem Lab Med. 2002 Dec;40(12):1183-90. PMID:12553418 doi:http://dx.doi.org/10.1515/CCLM.2002.208
- ↑ Saraiva MJ. Transthyretin mutations in health and disease. Hum Mutat. 1995;5(3):191-6. PMID:7599630 doi:http://dx.doi.org/10.1002/humu.1380050302
- ↑ Wojtczak A, Luft J, Cody V. Mechanism of molecular recognition. Structural aspects of 3,3'-diiodo-L-thyronine binding to human serum transthyretin. J Biol Chem. 1992 Jan 5;267(1):353-7. PMID:1730601