Transthyretin
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
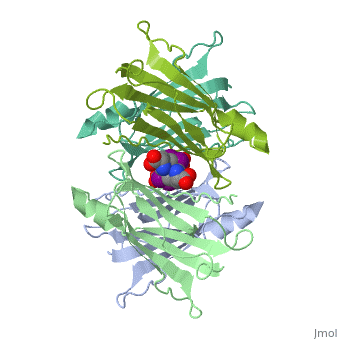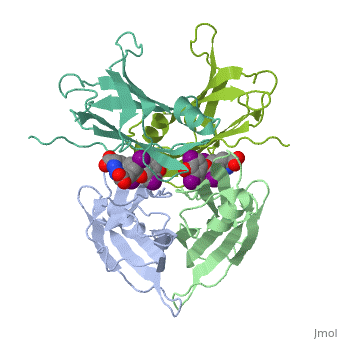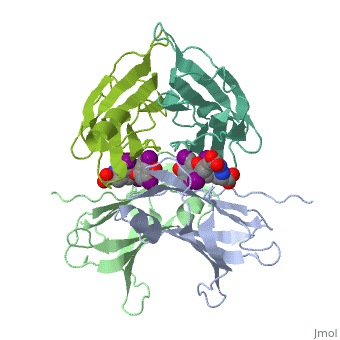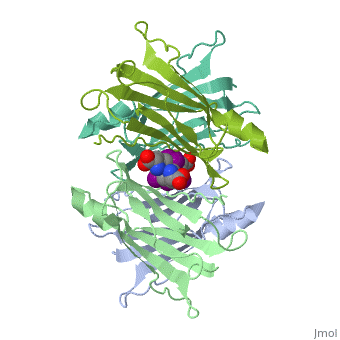
<StructureSection load='1tha' size='350' side='right' caption='Human transthyretin complex with tyroxine derivative [[1tha]]' scene='' pspeed='8'> | <StructureSection load='1tha' size='350' side='right' caption='Human transthyretin complex with tyroxine derivative [[1tha]]' scene='' pspeed='8'> | ||
== Function == | == Function == | ||
| - | '''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. | + | '''Transthyretin''' (TTR) is a serum carrier of the thyroid hormone thyroxine (T4) and retinol through its association with retinol-binding protein (RBP). Many small molecules bind to TTR T4-binding site<ref>PMID:12553418</ref>. For details see [[Tafamidis]] and [[Student Project 2 for UMass Chemistry 423 Spring 2015]]. |
== Disease == | == Disease == | ||
| - | TTR mutations are associated with amyloid deposition<ref>PMID:7599630</ref>. | + | TTR mutations are associated with amyloid deposition<ref>PMID:7599630</ref>. [[Tafamidis]] is a medication which stabilises TTR and is used in treatment of TTR amyloidosis<ref>PMID:30145929</ref>. |
== Structural highlights == | == Structural highlights == | ||
| Line 13: | Line 13: | ||
</StructureSection> | </StructureSection> | ||
| - | ==3D structures of transthyretin== | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *Transthyretin | ||
| - | |||
| - | **[[2pab]], [[1tta]], [[1ttb]], [[1etb]], [[1bmz]], [[1f41]], [[1dvq]], [[1fh2]], [[1qwh]], [[2h4e]], [[2qgb]], [[3cbr]], [[3d7p]], [[3cfm]], [[3i9p]], [[3a4d]], [[3a4e]], [[3a4f]], [[3u2i]], [[3w3b]], [[4mrb]], [[4pvl]], [[4pvn]], [[5k1j]], [[5k1n]], [[5cn3]], [[5cnh]], [[5dwp]], [[4tlt]], [[5lll]], [[5n7c]], [[5n62]], [[5n5q]], [[3ssg]], [[1zcr]], [[1zd6]] – hTTR – human<br /> | ||
| - | **[[3u2j]], [[4pvm]] – hTTR - neutron<br /> | ||
| - | **[[1eta]], [[1ttc]], [[1tsh]], [[2trh]], [[2try]], [[1bzd]], [[1bze]], [[1fhn]], [[1g1o]], [[1x7s]], [[2qel]], [[3dgd]], [[3did]], [[3kgs]], [[3tfb]] – hTTR (amyloidogenic mutant)<br /> | ||
| - | **[[6sdz]] - hTTR (amyloidogenic mutant) – Cryo EM<br /> | ||
| - | **[[1ttr]] – hTTR (cardiomyapatic mutant) | ||
| - | |||
| - | **[[1bz8]], [[5ttr]], [[1f86]], [[1gko]], [[1iii]], [[1iik]], [[1ijn]], [[1sok]], [[1soq]], [[1x7t]], [[2g3x]], [[2g3z]], [[2g4e]], [[2g4g]], [[2noy]], [[3bt0]], [[3cxf]], [[3djr]], [[3djs]], [[3djt]], [[3djz]], [[3dk0]], [[3dk2]], [[3do4]], [[3gps]], [[3grb]], [[3grg]], [[3i9a]], [[3i9i]], [[4mrc]], [[4pwe]], [[5dej]], [[5clx]], [[5clx]], [[5clz]], [[5cm1]], [[4tlu]], [[4tnf]], [[4tng]], [[4tkw]], [[4tl4]], [[4tl5]], [[4tlk]], [[4tls]], [[4tm9]], [[4tne]], [[5llv]], [[5h0z]], [[5h0y]], [[5h0x]], [[5h0w]], [[5h0v]], [[5fw8]], [[5fw7]], [[5fw6]], [[5fo2]], [[5cly]], [[6fzl]], [[6fxu]], [[6fwd]], [[6e71]], [[6e75]], [[6e76]], [[5oq0]], [[5nfw]], [[5nfe]] – hTTR (mutant)<br /> | ||
| - | **[[2nbp]], [[2nbo]] – hTTR (mutant) – NMR<br /> | ||
| - | **[[2qpf]] – TTR - mouse | ||
| - | |||
| - | **[[1gke]] – rTTR – rat<br /> | ||
| - | **[[1tfp]] – TTR – chicken<br /> | ||
| - | **[[1oo2]], [[1sn2]] – sbTTR – sea bream<br /> | ||
| - | **[[3uaf]] – TTR-52 (mutant) – ''Caenorhabditis elegans''<br /> | ||
| - | |||
| - | *Transthyretin binary complexes | ||
| - | |||
| - | **[[1thc]], [[2h1x]], [[2h6u]] – hTTR + hydroxyaurone derivative<br /> | ||
| - | **[[4pm1]] - hTTR + estradiol derivative<br /> | ||
| - | **[[1tlm]] – hTTR + milrinone | ||
| - | |||
| - | **[[1bm7]], [[3ozl]] – hTTR + flufenamic acid | ||
| - | |||
| - | **[[1dvs]], [[1dvt]], [[1dvu]], [[1dvx]], [[1dvy]], [[1dvz]], [[1tt6]], [[1tz8]], [[2f8i]], [[2fbr]], [[2flm]], [[2g5u]], [[2g9k]], [[2gab]], [[2qgc]], [[2qgd]], [[2qge]], [[3cn0]], [[3cn1]], [[3cn2]], [[3cn3]], [[3cn4]], [[3esn]], [[3eso]], [[3esp]], [[3glz]], [[3gs0]], [[3gs4]], [[3gs7]], [[3imr]], [[3ims]], [[3imt]], [[3imu]], [[3imv]], [[3imw]], [[3ipb]], [[3ipe]], [[3m10]], [[2fbr]], [[2flm]], [[3m1o]], [[3tct]], [[4fi7]], [[4fi8]], [[4fi6]], [[5tzl]], [[6e6z]] – hTTR + amyloid disease inhibitor<br /> | ||
| - | **[[4his]], [[4hiq]], [[6imx]], [[6imy]], [[6e72]], [[6e74]], [[6e77]] - hTTR (mutant) + amyloid disease inhibitor<br /> | ||
| - | **[[6fft]] - hTTR (mutant) + amyloid disease inhibitor - Neutron<br /> | ||
| - | **[[4abq]], [[4abv]], [[4abw]], [[4ac2]], [[4ac4]], [[4act]], [[4ank]], [[4abu]] – hTTR + ligand<br /> | ||
| - | **[[4des]], [[4det]], [[4der]], [[4dew]], [[4deu]] – hTTR + flavonoid<br /> | ||
| - | **[[1u21]], [[3cfn]], [[3cfq]], [[3cft]], [[3hj0]], [[3nee]], [[3neo]], [[3p3r]], [[3p3s]], [[3p3t]], [[3p3u]], [[4ky2]], [[4mas]], [[5e23]], [[5e4a]], [[5e4o]], [[5ezp]], [[5ayt]], [[4ydm]], [[4ydn]], [[4tq8]], [[4tqh]], [[4tqi]], [[4tqp]], [[5jim]], [[5jid]], [[6gr7]], [[6ep1]], [[5u4g]] – hTTR + inhibitor<br /> | ||
| - | **[[3nes]], [[3nex]] – hTTR (mutant) + inhibitor<br /> | ||
| - | **[[1e3f]], [[1e4h]], [[1e5a]], [[2b15]], [[5hjg]], [[5l4j]] – hTTR + phenol derivative<br /> | ||
| - | **[[4l1s]] - hTTR + benzamide derivative<br /> | ||
| - | **[[4l1t]] - hTTR + benzoate derivative<br /> | ||
| - | **[[5jiq]] - hTTR + benzophenone derivative<br /> | ||
| - | **[[5l4m]], [[6grp]] - hTTR + pyridine derivative<br /> | ||
| - | **[[4n85]], [[4n86]] - hTTR + glabridin<br /> | ||
| - | **[[4n87]] - hTTR (mutant) + glabridin<br /> | ||
| - | **[[2b14]], [[2b16]] – hTTR (mutant) + phenol derivative<br /> | ||
| - | **[[6r66]], [[6r67]], [[6r68]] - hTTR + flurbiprofen analog<br /> | ||
| - | **[[6r6i]] - hTTR (mutant) + flurbiprofen analog<br /> | ||
| - | **[[3kgt]], [[3kgu]], [[5akv]] – hTTR (mutant) + genistein derivative | ||
| - | |||
| - | **[[3ng5]] – hTTR (mutant) + epigallocatechin gallate | ||
| - | |||
| - | **[[1tyr]] – hTTR + retinoic acid | ||
| - | |||
| - | **[[1z7j]] - hTTR + tetraiodotyroacetic acid | ||
| - | |||
| - | **[[1y1d]], [[2b77]], [[2b9a]], [[3d2t]], [[3fc8]], [[3fcb]], [[2f7i]], [[4ikl]], [[4ikk]], [[4ikl]], [[4iki]], [[4ik7]], [[4ik6]], [[4iiz]], [[4ikj]], [[5l4i]], [[6e70]], [[6d0w]] – hTTR + NSAID drug<br /> | ||
| - | **[[4i89]], [[6e78]], [[6e73]] - hTTR (mutant) + NSAID drug<br /> | ||
| - | **[[4i87]], [[4i85]] - hTTR (mutant) + fibrillogenesis inhibitor <br /> | ||
| - | **[[3b56]] – hTTR + salicylic acid derivative<br /> | ||
| - | **[[4hjs]], [[4hjt]], [[4hju]] – hTTR + covalent kinetic stabilizer<br /> | ||
| - | **[[4pmf]] - hTTR + curcumin<br /> | ||
| - | **[[4qxv]], [[5en3]], [[5ihh]] - hTTR + luteolin<br /> | ||
| - | **[[4qya]] - hTTR (mutant) + luteolin<br /> | ||
| - | **[[4pwf]] - hTTR (mutant) + ferulic esterase<br /> | ||
| - | **[[4pwg]], [[4pwh]], [[4qrf]] - hTTR (mutant) + caffeic esterase<br /> | ||
| - | **[[4pwi]] - hTTR (mutant) + rosamariic acid<br /> | ||
| - | **[[4pwj]], [[4pwk]] - hTTR (mutant) + propolis<br /> | ||
| - | **[[4d7b]] - hTTR + tocalpone<br /> | ||
| - | **[[5a6i]] - hTTR (mutant) + tocalpone<br /> | ||
| - | **[[5aks]], [[5akt]], [[5al0]]- hTTR + resveratrol<br /> | ||
| - | **[[5al8]] - hTTR + daidzein glucuronide<br /> | ||
| - | **[[4y9b]], [[4y9c]], [[4y9e]], [[4y9f]], [[4y9g]] - hTTR (mutant) + mangostin<br /> | ||
| - | **[[4wnj]] - hTTR + quercetin<br /> | ||
| - | **[[5l4f]] - hTTR + cresol derivative<br /> | ||
| - | **[[4wns]], [[5u48]], [[5u49]], [[5u4a]], [[5u4b]], [[5u4c]], [[5u4d]], [[5u4e]], [[5u4f]] [[5u4g]] - hTTR + stilbene derivative<br /> | ||
| - | **[[4wo0]] - hTTR + apigenin<br /> | ||
| - | **[[5boj]] - hTTR + gemfibrozil<br /> | ||
| - | **[[1kgi]] – rTTR + tetraiodotyroacetic acid<br /> | ||
| - | **[[1kgj]] – rTTR + dibromoflavone<br /> | ||
| - | **[[6gnm]] - sbTTR + benzophenone derivative<br /> | ||
| - | **[[6gno]], [[6gnw]] - sbTTR + phenol derivative <br /> | ||
| - | **[[6gnp]], [[6gnr]] - sbTTR + pyridine derivative <br /> | ||
| - | **[[6gon]], [[6goo]] - sbTTR + perfluorooctanoate <br /> | ||
| - | |||
| - | *Transthyretin ternary complexes | ||
| - | |||
| - | **[[4pme]] - hTTR + ferulic acid + curcumin<br /> | ||
| - | **[[5cr1]]- hTTR + resveratrol + T4<br /> | ||
| - | |||
| - | *Transthyretin complexes with retinol-binding protein | ||
| - | |||
| - | **[[1rlb]], [[1qab]] – hTTR + RBP | ||
| - | |||
| - | **[[2wqa]] – hTTR + RBP + oleic acid | ||
| - | |||
| - | **[[3bsz]] – hTTR + RBP + antibody | ||
| - | |||
| - | *Transthyretin complexes with thyroxine | ||
| - | |||
| - | **[[2rox]], [[1ict]] – hTTR + T4 | ||
| - | |||
| - | **[[3ozk]] – hTTR (mutant) + T4 | ||
| - | |||
| - | **[[1tha]], [[2roy]] – hTTR + T4 derivative | ||
| - | |||
| - | **[[1ie4]] – rTTR + T4 | ||
| - | |||
| - | **[[1sn0]] – gsTTR + T4 | ||
| - | |||
| - | **[[1sn5]] – gsTTR + T4 derivative | ||
| - | |||
| - | *Transthyretin peptide amyloid forming | ||
| - | |||
| - | **[[6c3f]], [[6c3g]], [[6c3s]], [[6c3t]], [[6c4o]], [[6c88]], [[4xfn]], [[4xfo]] - hTTR <br /> | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Robbins J. Transthyretin from discovery to now. Clin Chem Lab Med. 2002 Dec;40(12):1183-90. PMID:12553418 doi:http://dx.doi.org/10.1515/CCLM.2002.208
- ↑ Saraiva MJ. Transthyretin mutations in health and disease. Hum Mutat. 1995;5(3):191-6. PMID:7599630 doi:http://dx.doi.org/10.1002/humu.1380050302
- ↑ Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018 Sep 13;379(11):1007-1016. PMID:30145929 doi:10.1056/NEJMoa1805689
- ↑ Wojtczak A, Luft J, Cody V. Mechanism of molecular recognition. Structural aspects of 3,3'-diiodo-L-thyronine binding to human serum transthyretin. J Biol Chem. 1992 Jan 5;267(1):353-7. PMID:1730601