Sialyltransferase
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> == Function == Sialyltransferase (SIT) is involved in maintaining or increasing cell ...) |
|||
| (22 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='2x61' size='350' side='right' caption='α-2,3-sialyltransferase complex with trisaccharide, acetate, MPD and ethylene glycol (PDB code [[2x61]])' scene='74/748880/Cv/7'> | |
| - | <StructureSection load=' | + | |
== Function == | == Function == | ||
| - | Sialyltransferase (SIT) is involved in maintaining or increasing cell surface sialysation which | + | Sialyltransferase (SIT) is involved in maintaining or increasing cell surface sialysation which contributes to adhesive cellular interactions and thus to the growth and differentiation of hematopoietic progenitor cells<ref>PMID:15750786</ref>. Humans contain more than 20 different SIT differing in their substrate specificity, tissue distribution and various biochemical parameters<ref>PMID:11530204</ref>. |
| - | + | *'''Alpha-2,3-sialyltransferase''', '''Alpha-2,6-sialyltransferase''', '''Alpha-2,8-sialyltransferase''' catalyze the transfer of sialyl from a CMP-linked sialic acid donor to a terminal alpha-2,3, alpha-2,6, alpha-2,8-linked sialic acid of N-linked oligosaccharides of olygoproteins<ref>PMID:10766765</ref>,<ref>PMID:26192331</ref>,<ref>PMID:9826427</ref> . | |
| - | + | *'''Beta-galactoside alpha-2,6-sialyltransferase''' performs the final glycosylation in many glycoproteins by transferring a sialyl to a terminal galactose<ref>PMID:23999306</ref>. | |
== Relevance == | == Relevance == | ||
| Line 11: | Line 10: | ||
== Structural highlights == | == Structural highlights == | ||
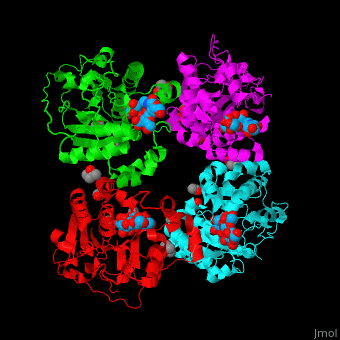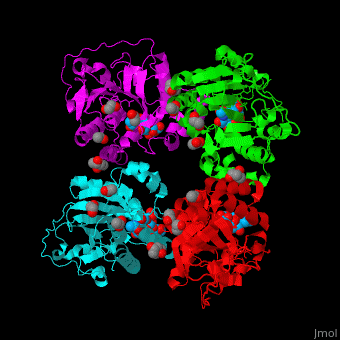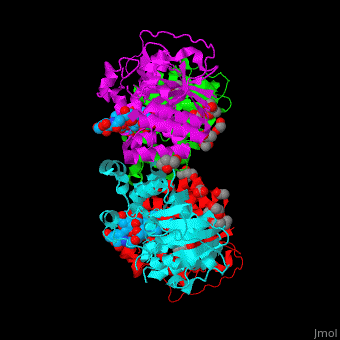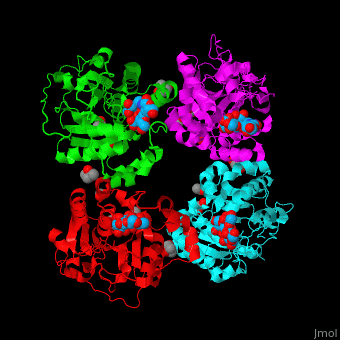
| + | The biological assembly of α-2,3-sialyltransferase is <scene name='74/748880/Cv/8'>homotetramer</scene> ([[2x61]]). The <scene name='74/748880/Cv/9'>trisaccharide acceptor binds to the open cleft</scene> at the C-terminal of SIT<ref>PMID:21832050</ref>. <scene name='74/748880/Cv/10'>Trisaccharide binding site</scene>. Water molecules are shown as red spheres. | ||
| + | ==3D structures of sialyltransferase== | ||
| + | [[Sialyltransferase 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| - | ==3D structures of sialyltransferase== | ||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Schwartz-Albiez R, Merling A, Martin S, Haas R, Gross HJ. Cell surface sialylation and ecto-sialyltransferase activity of human CD34 progenitors from peripheral blood and bone marrow. Glycoconj J. 2004;21(8-9):451-9. PMID:15750786 doi:http://dx.doi.org/10.1007/s10719-004-5535-5
- ↑ Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001 Aug;83(8):727-37. PMID:11530204
- ↑ Angata K, Suzuki M, McAuliffe J, Ding Y, Hindsgaul O, Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem. 2000 Jun 16;275(24):18594-601. PMID:10766765 doi:http://dx.doi.org/10.1074/jbc.M910204199
- ↑ Volkers G, Worrall LJ, Kwan DH, Yu CC, Baumann L, Lameignere E, Wasney GA, Scott NE, Wakarchuk W, Foster LJ, Withers SG, Strynadka NC. Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation. Nat Struct Mol Biol. 2015 Jul 20. doi: 10.1038/nsmb.3060. PMID:26192331 doi:http://dx.doi.org/10.1038/nsmb.3060
- ↑ Lee YC, Kim YJ, Lee KY, Kim KS, Kim BU, Kim HN, Kim CH, Do SI. Cloning and expression of cDNA for a human Sia alpha 2,3Gal beta 1, 4GlcNA:alpha 2,8-sialyltransferase (hST8Sia III). Arch Biochem Biophys. 1998 Dec 1;360(1):41-6. PMID:9826427 doi:http://dx.doi.org/10.1006/abbi.1998.0909
- ↑ Kuhn B, Benz J, Greif M, Engel AM, Sobek H, Rudolph MG. The structure of human alpha-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr D Biol Crystallogr. 2013 Sep;69(Pt 9):1826-38. doi:, 10.1107/S0907444913015412. Epub 2013 Aug 17. PMID:23999306 doi:http://dx.doi.org/10.1107/S0907444913015412
- ↑ Wang L, Liu Y, Wu L, Sun XL. Sialyltransferase inhibition and recent advances. Biochim Biophys Acta. 2016 Jan;1864(1):143-53. doi: 10.1016/j.bbapap.2015.07.007., Epub 2015 Jul 18. PMID:26192491 doi:http://dx.doi.org/10.1016/j.bbapap.2015.07.007
- ↑ Lee HJ, Lairson LL, Rich JR, Lameignere E, Wakarchuk WW, Withers SG, Strynadka NC. Structural and kinetic analysis of substrate binding to the sialyltransferase CST-II from Campylobacter Jejuni. J Biol Chem. 2011 Aug 8. PMID:21832050 doi:10.1074/jbc.M111.261172