User:Kyle Burton/Sandbox1
From Proteopedia
< User:Kyle Burton(Difference between revisions)
| (37 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='1b7f' size='350' side='right' caption='Sex-Lethal protein' scene='78/783145/Full_structure/7'> | <StructureSection load='1b7f' size='350' side='right' caption='Sex-Lethal protein' scene='78/783145/Full_structure/7'> | ||
== Background == | == Background == | ||
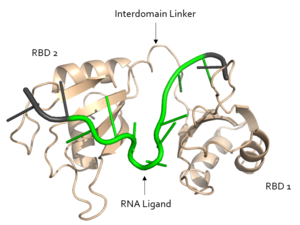
| - | + | '''Sex Lethal Protein''' (Sxl) is an important splicing repressor in the male developmental pathway and [https://en.wikipedia.org/wiki/Sex-determination_system sex determination] of the common fruit fly, ''[https://en.wikipedia.org/wiki/Drosophila_melanogaster Drosophila melanogaster]''<ref name="Handa">PMID: 10217141</ref>. Sxl regulates [https://en.wikipedia.org/wiki/Alternative_splicing alternative splicing] pathways to promote the expression of female sex-linked proteins. In eukaryotes, splicing is carried out via the [https://en.wikipedia.org/wiki/Spliceosome spliceosome], a [https://en.wikipedia.org/wiki/Ribozyme ribozyme]-protein complex which binds to the 5’ and 3’ splice sites. Sxl prevents the binding of the [https://en.wikipedia.org/wiki/U2AF2 U2AF] and [https://en.wikipedia.org/wiki/U1_spliceosomal_RNA U1 subunits] of the spliceosome at their respective splice sites, which represses their alternative splicing pathways<ref name="Penalva">Penalva L, Sanchez L. RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation. Microbiol Mol Biol Rev.;67(3):343-356. doi: 10.1128/MMBR.67.3.343–359.2003</ref>. As a result, the fruit fly expressing ''Sxl'' will produce mRNA transcripts encoding proteins for the female developmental pathway<ref name="Handa"/>. [[Image:Sex Lethal Protein Structural Overview with Labels.png|300px|right|thumb| '''Figure 1.''' Structural overview of Sxl. RNA ligand colored in green is recognized and bound, RNA ligand colored in grey is not bound. Structure shown is [https://www.rcsb.org/structure/1b7f PDB:1b7f]. Figure created in PyMol.]] | |
| - | '''Sex Lethal Protein''' (Sxl) is an important splicing repressor in the male developmental pathway and [https://en.wikipedia.org/wiki/Sex-determination_system sex determination] of the common fruit fly, ''[https://en.wikipedia.org/wiki/Drosophila_melanogaster Drosophila melanogaster]''<ref name="Handa">PMID: 10217141</ref>. Sxl regulates [https://en.wikipedia.org/wiki/Alternative_splicing alternative splicing] pathways to promote the expression of female sex-linked proteins. In eukaryotes, splicing is carried out via the [https://en.wikipedia.org/wiki/Spliceosome spliceosome], a [https://en.wikipedia.org/wiki/Ribozyme ribozyme]-protein complex which binds to the 5’ and 3’ splice sites. Sxl prevents the binding of the [https://en.wikipedia.org/wiki/U2AF2 U2AF] and [https://en.wikipedia.org/wiki/U1_spliceosomal_RNA U1 subunits] of the spliceosome at their respective splice sites, which represses their alternative splicing pathways<ref name="Penalva">Penalva L, Sanchez L. RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation. Microbiol Mol Biol Rev.;67(3):343-356. doi: 10.1128/MMBR.67.3.343–359.2003</ref>. As a result, the fruit fly expressing ''Sxl'' will produce mRNA transcripts encoding proteins for the female developmental pathway<ref name="Handa"/>. | + | |
| - | Sxl targets the ''transformer'' (''tra'') and ''msl-2'' primary transcripts. Tra is a splicing activator for the female developmental pathway, and ''msl-2'' expression modulates [https://en.wikipedia.org/wiki/X_chromosome X chromosome] application in male fruit flies<ref name="Bashaw">PMID: 7588059</ref>. If Sxl is unable to repress translation of the male-sex lethal (Msl-2) protein in female flies, the female fly will die due to hyperexpression of both X chromosomes<ref name="Black">doi: 10.1146/annurev.biochem.72.121801.161720</ref><ref name="Georgiev">PMID: 21339706</ref>. The mechanism for how Sxl targets these pathways differs slightly. In both mechanisms, Sxl occupies the 3' splice site and prevents [https://en.wikipedia.org/wiki/U2AF2 U2AF] from binding. This causes the U2AF splicing factor to bind at a downstream splice site encoding proteins in the female developmental pathway. In ''msl-2'' targeting, Sxl also blocks the binding of another regulatory splicing factor, Rox8, and the [https://en.wikipedia.org/wiki/SnRNP U1 snRNP] at the 5’ splice site<ref name="Penalva"/>. Sxl can also control its own splicing pattern to conserve female expression. It does so by binding to [https://en.wikipedia.org/wiki/Exon Exon] 3 of its own RNA and creating an RNP complex to eliminate this exon. After removal of Exon 3, Sxl becomes active and female expression is maintained. | + | Sxl targets the ''transformer'' (''tra'') and ''msl-2'' primary transcripts. Tra is a splicing activator for the female developmental pathway, and ''msl-2'' expression modulates [https://en.wikipedia.org/wiki/X_chromosome X chromosome] application in male fruit flies<ref name="Bashaw">PMID: 7588059</ref><ref name="Kelley">PMID: 7781064</ref>. If Sxl is unable to repress translation of the male-sex lethal (Msl-2) protein in female flies, the female fly will die due to hyperexpression of both X chromosomes<ref name="Black">doi: 10.1146/annurev.biochem.72.121801.161720</ref><ref name="Georgiev">PMID: 21339706</ref>. The mechanism for how Sxl targets these pathways differs slightly. In both mechanisms, Sxl occupies the 3' splice site and prevents [https://en.wikipedia.org/wiki/U2AF2 U2AF] from binding. This causes the U2AF splicing factor to bind at a downstream splice site encoding proteins in the female developmental pathway. In ''msl-2'' targeting, Sxl also blocks the binding of another regulatory splicing factor, Rox8, and the [https://en.wikipedia.org/wiki/SnRNP U1 snRNP] at the 5’ splice site<ref name="Penalva"/>. Sxl can also control its own splicing pattern to conserve female expression. It does so by binding to [https://en.wikipedia.org/wiki/Exon Exon] 3 of its own RNA and creating an RNP complex to eliminate this exon. After removal of Exon 3, Sxl becomes active and female expression is maintained. |
== Structure == | == Structure == | ||
| - | |||
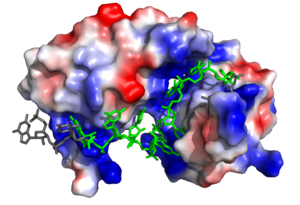
[[Image:Sex lethal protein electrostatic surface representation.png|300px|left|thumb| '''Figure 2.''' Sxl showing the electropositive binding pocket and the bound RNA ligand. Pre-mRNA residues binding to Sxl shown in green, non-binding residues shown in grey. Structure shown is [https://www.rcsb.org/structure/1b7f PDB:1b7f]. Figure created in PyMol.]] | [[Image:Sex lethal protein electrostatic surface representation.png|300px|left|thumb| '''Figure 2.''' Sxl showing the electropositive binding pocket and the bound RNA ligand. Pre-mRNA residues binding to Sxl shown in green, non-binding residues shown in grey. Structure shown is [https://www.rcsb.org/structure/1b7f PDB:1b7f]. Figure created in PyMol.]] | ||
| - | Sxl is composed of two asymmetric RNA binding domains (RBD1 and RBD2) which recognize a poly-uridine site in the pre-mRNA transcript<ref name="Handa"/>. | + | Sxl is composed of two asymmetric RNA binding domains (RBD1 and RBD2) which recognize a poly-uridine site in the pre-mRNA transcript<ref name="Handa"/>. <scene name='78/783145/Secondary_structure/3'>Both RBDs</scene> are comprised of two [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] and one antiparallel four-stranded [https://en.wikipedia.org/wiki/Beta_sheet β sheet]<ref name="Handa"/> containing the [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motif](Fig. 1). The β sheets face each other, lining the V-shaped cleft<ref name="Handa"/>, shown in sand in Fig. 1. The inter-domain linker, shown in sand in Fig. 1, forms a distorted 3<sub>10</sub> helix which helps form the V-shaped cleft into which the pre-mRNA sequence binds<ref name="Handa"/><ref name="Black">doi: 10.1146/annurev.biochem.72.121801.161720</ref>. Sxl binds to UGUUUUUUU sequence of GUUGUUUUUUUU in the ''tra'' pre-mRNA<ref name="Handa"/><ref name="Black"/>. RBD1 binds U6-U11 and RBD2 binds U3, G4, and U5. Figure 1 shows bound pre-mRNA residues in green and non-bound pre-mRNA residues in grey. Although the two RBDs do not interact with each other, this nine-ribonucleotide sequence must be recognized continuously to allow Sxl to bind, preventing U2AF from binding at the 3’ splice site<ref name="Handa"/>. The binding of Sxl to the pre-mRNA occurs in an electropositive pocket (shown in blue in Fig. 2) due to extensive interactions with the RNA phosphate backbone and negatively charged residues<ref name="Handa"/>. Because non-RBD residues are not essential to Sxl's function, there is some variation in the ''sxl'' gene of other drosopholids, though the RBD residues are highly conserved among all drosopholids<ref name="Penalva"/>. |
| - | + | ||
=== Structural Basis for Recognition of Poly-U Sequences === | === Structural Basis for Recognition of Poly-U Sequences === | ||
| - | The structural interactions with regards to the targeting of the 5' splice site and of its own mRNA transcript are much less understood than the competition of <scene name='78/783145/Sxl/ | + | The structural interactions with regards to the targeting of the 5' splice site and of its own mRNA transcript are much less understood than the competition of <scene name='78/783145/Sxl/2'>Sxl</scene> with U2AF at the 3' splice site. All the RNA-protein interactions described here refer to ''tra''-Sxl interactions<ref name="Handa"/>. There are no published crystal structures of the Sxl-''msl-2'' complex, but Sxl recognizes the same poly-U site in both ''tra'' and ''msl-2''. |
| - | The <scene name='78/783145/Arg_252_interaction_with_u3_g4/6'>R252 interaction with U3 and G4</scene> is crucial to pre-mRNA binding; a mutation of R252 to alanine eliminated the ability of Sxl to bind RNA<ref name=" | + | The <scene name='78/783145/Arg_252_interaction_with_u3_g4/6'>R252 interaction with U3 and G4</scene> is crucial to pre-mRNA binding; a mutation of R252 to alanine eliminated the ability of Sxl to bind RNA<ref name="original">PMID: 9398148</ref>. |
| - | The ligand pre-mRNA sequence forms a <scene name='78/783145/U5_u6_u7_loop/5'>loop</scene> | + | The ligand pre-mRNA sequence forms a <scene name='78/783145/U5_u6_u7_loop/5'>loop</scene> at U5, U6, and U7. This interaction is stabilized by π stacking between G4 and U5 of the pre-mRNA ligand and <scene name='78/783145/Aromatic_stacking/5'>Y214 and F256</scene>, respectively. The nucleobases are exposed to residues on Sxl due to the 2’ endo conformation of all the nucleotides except for U8, which maintains a 3’ endo conformation. |
| - | The U6 residue is recognized as part of the RNA <scene name='78/783145/U5_u6_u7_loop/13'>loop</scene> by R195. The R195 amide hydrogen-bonds to the O2' of U6 and the U6 N3H hydrogen bonds to the R195 carbonyl oxygen | + | The U6 residue is recognized as part of the RNA <scene name='78/783145/U5_u6_u7_loop/13'>loop at U5, U6, and U7</scene> by R195. The R195 amide hydrogen-bonds to the O2' of U6 and the U6 N3H hydrogen bonds to the R195 carbonyl oxygen. |
| - | In the | + | In the <scene name='78/783145/U5_u6_u7_loop/14'>RNA loop</scene>, the U7 and U8 bases are involved in <scene name='78/783145/U7_u8_stacking/3'>π stacking</scene>, stabilizing the 3' endo conformation of the U8 sugar. U8 is further stabilized via hydrogen bonding <scene name='78/783145/U8_with_s165_and_y166/5'>interactions with S165 and Y166</scene>, where the nitrogens of the uridine ring hydrogen bond to the respective carbonyl oxygens of S165 and Y166. |
| - | <scene name='78/783145/N130_interaction_with_u9/ | + | <scene name='78/783145/N130_interaction_with_u9/5'>U9</scene> is recognized by the interdomain linker. This interaction is permissible due to free rotation in the N130 side chain, allowing hydrogen bonding between the N130 side chain and a phosphate oxygen of U9. U9 is further stabilized by a second <scene name='78/783145/U9_with_interdomain_linker/1'>an ion-dipole interaction</scene> between the U9 O2' and the side chain of R202 and the U9 O4' and the K197 side chain. |
| - | U9 facilitates the stabilization of U10, which is also recognized by the interdomain linker. <scene name='78/783145/Arg_258_interaction_w_u9_u10/3'>R258 interacts with U9 and U10</scene> to form a salt bridge. | + | U9 facilitates the stabilization of U10, which is also recognized by the interdomain linker. A phosphate oxygen of <scene name='78/783145/Arg_258_interaction_w_u9_u10/3'>R258 interacts with U9 and U10</scene> to form a [https://en.wikipedia.org/wiki/Salt_bridge salt bridge]. |
| - | U11 is recognized by R155. The O2' of U11 <scene name='78/783145/R155_intxn_with_u11/3'>interacts with R155</scene> to form a hydrogen bond | + | U11 is recognized by R155. The O2' of U11 <scene name='78/783145/R155_intxn_with_u11/3'>interacts with R155</scene> to form a hydrogen bond. |
| - | The above interactions are relevant in that Sxl recognizes the specific pre-mRNA based mostly on interactions with the sugar-phosphate backbones<ref name="Handa"/>. Many proteins with [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] are specific in the interactions they form with the bases of the RNA recognized. In contrast, Sxl has a high specificity despite primarily interacting with the phosphate backbone. | + | The above interactions are relevant in that Sxl recognizes the specific pre-mRNA based mostly on interactions with the sugar-phosphate backbones<ref name="Handa"/>. Many proteins with [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] are specific in the interactions they form with the bases of the RNA recognized<ref name="Black"/>. In contrast, Sxl has a high specificity despite primarily interacting with the phosphate backbone. |
== Alternative Splicing Pathways == | == Alternative Splicing Pathways == | ||
| Line 36: | Line 33: | ||
=== Autoregulation === | === Autoregulation === | ||
| - | Sxl is capable of autoregulation of its expression<ref name="Black"/>. Sxl protein autoregulates negatively by binding the poly-U sequence in its own 5'UTR, repressing translation<ref name="Black"/>. The negative autoregulation results in maintaining appropriate concentrations of Sxl. Sxl can also positively autoregulate its expression: the ''Sxl'' gene is transcribed in male flies, but the inclusion of exon 3 results in a premature stop codon, producing an inactive, truncated protein<ref name="Black"/>. The same ''Sxl'' [https://en.wikipedia.org/wiki/Promoter_(genetics) promoter] is active in female flies, but an additional (briefly active) ''Sxl'' promoter produces a transcript with exon 3 removed, resulting in an active Sxl protein which will initiate other female-specific splicing cascades<ref name="Black"/>. | + | Sxl is capable of autoregulation of its expression<ref name="Black"/><ref name="Bell">PMID: 2015624</ref>. Sxl protein autoregulates negatively by binding the poly-U sequence in its own 5'UTR, repressing translation<ref name="Black"/>. The negative autoregulation results in maintaining appropriate concentrations of Sxl. Sxl can also positively autoregulate its expression: the ''Sxl'' gene is transcribed in male flies, but the inclusion of exon 3 results in a premature stop codon, producing an inactive, truncated protein<ref name="Black"/><ref name="Bell"/>. The same ''Sxl'' [https://en.wikipedia.org/wiki/Promoter_(genetics) promoter] is active in female flies, but an additional (briefly active) ''Sxl'' promoter produces a transcript with exon 3 removed, resulting in an active Sxl protein which will initiate other female-specific splicing cascades<ref name="Black"/>. |
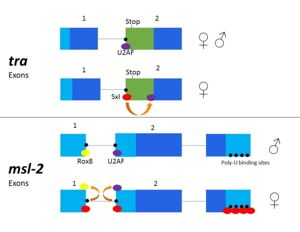
=== ''Tra'' === | === ''Tra'' === | ||
| Line 42: | Line 39: | ||
=== ''Msl-2'' === | === ''Msl-2'' === | ||
| - | + | Msl-2 is responsible for [https://en.wikipedia.org/wiki/Dosage_compensation dosage compensation] of X chromosomes in fruit flies<ref name="Bashaw"/><ref name="Kelley"/>. The alternative splicing of ''msl-2'' is reliant on Sxl binding to both the 5' and 3' splice sites (Fig. 3). Sxl binds at the 3' splice site, replacing U2AF as in ''tra'' splicing. Sxl also competes with [http://www.uniprot.org/uniprot/Q26281 Rox8], which binds to the first intron. As a result, Sxl prevents splicing of the first intron of the ''msl-2'' primary transcript<ref name="Georgiev"/>. Sxl also binds to the poly- U sequences of the 3' UTR to repress translation (Fig.3), leading to female expression<ref name="Kelley"/><ref>PMID: 9570314</ref><ref name="Penalva"/>. When Sxl targets ''msl-2'', the first intron is retained<ref name="Black"/><ref name="Georgiev"/>. However, the retained intron is in the [https://en.wikipedia.org/wiki/Untranslated_region 5' UTR] and does not affect the reading frame<ref name="Black"/>. When ''msl-2'' is expressed, the X chromosome's transcription is repressed. In male fruit flies, ''msl-2'' must be inactivated to allow increased X chromosome transcription<ref name="Georgiev"/>. As the expression of ''msl-2'' is exclusively required for male fruit fly development, any mutation in Sxl protein which causes splicing and activation of the ''msl-2'' gene in females leads to female fly death by hyperexpression of both X chromosomes<ref name="Georgiev"/>. | |
| - | Msl-2 is responsible for [https://en.wikipedia.org/wiki/Dosage_compensation dosage compensation] of X chromosomes in fruit flies<ref name="Bashaw"/>. The alternative splicing of ''msl-2'' is reliant on Sxl binding to both the 5' and 3' splice sites (Fig. 3). Sxl binds at the 3' splice site, replacing U2AF as in ''tra'' splicing. Sxl also competes with [http://www.uniprot.org/uniprot/Q26281 Rox8], which binds to the first intron. As a result, Sxl prevents splicing of the first intron of the ''msl-2'' primary transcript<ref name="Georgiev"/>. Sxl also binds to the poly- U sequences of the 3' UTR to repress translation (Fig.3)<ref name="Penalva"/> | + | |
== Relevance == | == Relevance == | ||
| - | As Sxl functions as a splicing repressor, it may give insight into the effects of varying mechanisms of alternate splicing both in flies and other species. Sxl may also lead to understanding of human alternative splicing factors. As an RNA binding protein, research regarding Sxl may contribute to the understanding of enzymes with RNA recognition motifs. The Sxl RNP motif of RBD1 is also conserved in the ELAV family of proteins<ref name="Handa"/>. Sxl is only one of many proteins which regulate dosage compensation, but is one that is observed in XX/XY systems, and thus could lead to better comprehension of dosage compensation in species with similar sex determination systems | + | As Sxl functions as a splicing repressor, it may give insight into the effects of varying mechanisms of alternate splicing both in flies and other species. Sxl may also lead to understanding of human alternative splicing factors. As an RNA binding protein, research regarding Sxl may contribute to the understanding of enzymes with RNA recognition motifs. The Sxl RNP motif of RBD1 is also conserved in the ELAV family of proteins<ref name="Handa"/><ref>PMID: 9299339</ref>. Sxl is only one of many proteins which regulate dosage compensation, but is one that is observed in XX/XY systems, and thus could lead to better comprehension of dosage compensation in species with similar sex determination systems<ref name="Georgiev"/><ref name="Kelley"/> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature. 1999 Apr 15;398(6728):579-85. PMID:10217141 doi:10.1038/19242
- ↑ 2.0 2.1 2.2 2.3 2.4 Penalva L, Sanchez L. RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation. Microbiol Mol Biol Rev.;67(3):343-356. doi: 10.1128/MMBR.67.3.343–359.2003
- ↑ 3.0 3.1 3.2 Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development. 1995 Oct;121(10):3245-58. PMID:7588059
- ↑ 4.0 4.1 4.2 4.3 Kelley RL, Solovyeva I, Lyman LM, Richman R, Solovyev V, Kuroda MI. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995 Jun 16;81(6):867-77. PMID:7781064
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291-336. doi: 10.1146/annurev.biochem.72.121801.161720., Epub 2003 Feb 27. PMID:12626338 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161720
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Georgiev P, Chlamydas S, Akhtar A. Drosophila dosage compensation: males are from Mars, females are from Venus. Fly (Austin). 2011 Apr-Jun;5(2):147-54. Epub 2011 Apr 1. PMID:21339706
- ↑ Lee AL, Volkman BF, Robertson SA, Rudner DZ, Barbash DA, Cline TW, Kanaar R, Rio DC, Wemmer DE. Chemical shift mapping of the RNA-binding interface of the multiple-RBD protein sex-lethal. Biochemistry. 1997 Nov 25;36(47):14306-17. doi: 10.1021/bi970830y. PMID:9398148 doi:http://dx.doi.org/10.1021/bi970830y
- ↑ 8.0 8.1 Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229-39. PMID:2015624
- ↑ Gebauer F, Merendino L, Hentze MW, Valcarcel J. The Drosophila splicing regulator sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA. RNA. 1998 Feb;4(2):142-50. PMID:9570314
- ↑ Inoue M, Muto Y, Sakamoto H, Kigawa T, Takio K, Shimura Y, Yokoyama S. A characteristic arrangement of aromatic amino acid residues in the solution structure of the amino-terminal RNA-binding domain of Drosophila sex-lethal. J Mol Biol. 1997 Sep 12;272(1):82-94. PMID:9299339 doi:10.1006/jmbi.1997.1213