A new modulated crystal structure of ANS complex of St John's wort Hyp-1 protein with 36 protein molecules in the asymmetric unit of the supercell
Joanna Smietanska, Joanna Sliwiak, Miroslaw Gilski, Zbigniew Dauter, Radoslaw Strzalka, Janusz Wolny, Mariusz Jaskolski [1]
Molecular Tour
Hyp-1 overall structure and function
Hyp-1 from St John’s wort (Hypericum perforatum) belongs to pathogenesis-related proteins of class 10 (PR-10). These ubiquitous plant proteins are expressed under stressful conditions, such as salinity, drought or pathogen invasion [2]. St. John’s wort is an important herb, widely used for millennia in folk medicine for the treatment of various ailments [3]. PR-10 proteins are among the most intriguing plant proteins because of the difficulty of assigning to them a unique biological function, despite their abundance, coexistence of many isoforms in one plant, and comprehensive structural characterization. The characteristic PR-10 fold consists of a seven-stranded antiparallel β-sheets wrapped around a long C-terminal helix α3, which rests on a V-shaped fork of two additional short helices α1 and α2. A progression of β-hairpins and loops connect the β-strands, except strands β1 and β2 which form the opposite edges of the β-sheet [4]. The PR-10 fold creates a large hydrophobic cavity between the β-sheet and helix α3 that is suggestive of a ligand binding function. A number of hydrophobic mediators have been already confirmed as PR-10 partners, most notably phytohormones such as cytokinins. Such complexes are usually studied using ANS Displacement Assays (ADA), in which the fluorescent dye 8-anilino-1-naphthalene sulfonate (ANS) is displaced by the ligand of interest [5]. In order to elucidate the molecular basis of this method we crystallized Hyp-1 in complex with ANS. Our first crystal turned out to be tetartohedrally twinned and have a modulated superstructure, which was interpreted in a sevenfold expanded unit cell containing 28 Hyp-1 molecules and 89 ANS ligands [2][3]. In the present study, we co-crystallized Hyp-1 with a mixture of ANS and melatonin, which was recently found to be a PR-10 ligand for cross-talk with other phytohormones [6]. To our surprise, melatonin was unable to displace ANS and instead we obtained a crystal of Hyp-1/ANS complex with an even more daunting modulation: 36 Hyp-1 and 156 ANS molecules in a ninefold expanded unit cell.
The phenomenon of superstructure modulation
Superstructure modulation is a crystal abnormality, where exact periodicity of the unit cells is violated, and the wave of distortion has a long period (running over many unit cells), which can be commensurate or incommensurate with the basic lattice periodicity. In reciprocal space, this phenomenon is manifested by the presence of weaker satellites dividing the spaces between the main Bragg reflections, according to a modulation vector q [7]. Superstructure modulation is well known in small-molecule crystallography but in macromolecular crystallography it is practically unheard of [8]. The first successful structure solution and refinement of a modulated protein crystal structure was presented for the Hyp-1/ANS with protein with sevenfold expansion of the C2 unit cell [2][3]. Here, we present the crystal structure of the same complex but with ninefold commensurate modulation. The asymmetric unit contains 36 independent Hyp-1 molecules and 156 ANS ligands. 95 ANS molecules occupy (with variable frequency) three binding sites of the Hyp-1 molecule, two of which are located within the familiar hydrophobic cavity, while the third one is formed as a deep surface invagination. The remaining 61 ANS ligands are bound on protein surfaces, gluing together neighboring protein molecules and generating the bizarre pattern of crystal packing.
Structural highlights of Hyp-1/ANS complexes
Preincubation of the Hyp-1 protein with an ANS:melatonin mixture and further crystallization trials resulted in a new crystal form of the Hyp-1/ANS complex, with ninefold noncrystallographic repetition of the same structural motif (two Hyp-1 dimers) along c.
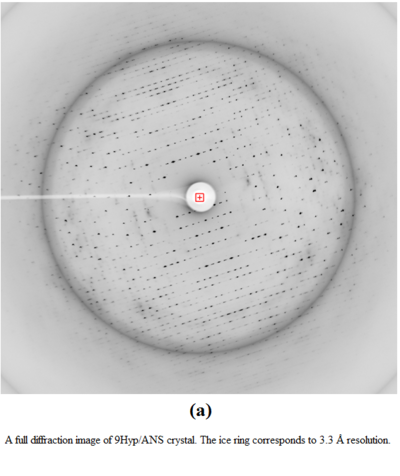
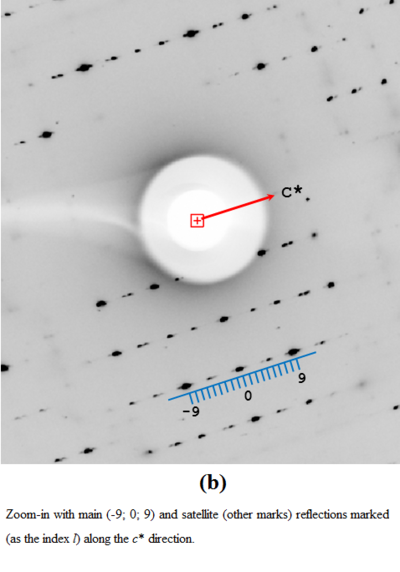
A physical manifestation of this superstructure modulation is the fluctuation of the diffraction pattern intensity, with strong reflections for the l index equal 9n, and also in-between, at l=9n±4.
Each Hyp-1 molecule harbors three internal ligand binding sites, which are variously populated by 95 ANS ligands in the 36 copies of the protein.
There are 61 ANS molecules bound on the surface of the Hyp-1 molecules, which are most likely the generator of superstructure modulation.
X-Ray diffraction images of 9Hyp/ANS crystal:
(labeled A, B,…Z, a, b,…j) in the asymmetric part of the expanded unit cell. The protein molecules are arranged with ninefold noncrystallographic repetition of the same structural motif (, e.g. AB and ij) along c (dashed line). Small-molecule ligands are marked in ball-and-stick representation.
. The main binding sites located in two internal chambers (1, 2) and in a deep surface pocket (3) display better ligand conformational stability than the interstitial sites 4-8.
PDB reference: Hyp-1–ANS complex with ninefold structure modulation, 6sjj
References
- ↑ Smietanska J, Sliwiak J, Gilski M, Dauter Z, Strzalka R, Wolny J, Jaskolski M. A new modulated crystal structure of the ANS complex of the St John's wort Hyp-1 protein with 36 protein molecules in the asymmetric unit of the supercell. Acta Crystallogr D Struct Biol. 2020 Jul 1;76(Pt 7):653-667. doi:, 10.1107/S2059798320006841. Epub 2020 Jun 17. PMID:32627738 doi:http://dx.doi.org/10.1107/S2059798320006841
- ↑ 2.0 2.1 2.2 Sliwiak J, Jaskolski M, Dauter Z, McCoy AJ, Read RJ. Likelihood-based molecular-replacement solution for a highly pathological crystal with tetartohedral twinning and sevenfold translational noncrystallographic symmetry. Acta Crystallogr D Biol Crystallogr. 2014 Feb;70(Pt 2):471-80. doi:, 10.1107/S1399004713030319. Epub 2014 Jan 29. PMID:24531481 doi:http://dx.doi.org/10.1107/S1399004713030319
- ↑ 3.0 3.1 3.2 Sliwiak J, Dauter Z, Kowiel M, McCoy AJ, Read RJ, Jaskolski M. ANS complex of St John's wort PR-10 protein with 28 copies in the asymmetric unit: a fiendish combination of pseudosymmetry with tetartohedral twinning. Acta Crystallogr D Biol Crystallogr. 2015 Apr;71(Pt 4):829-43. doi:, 10.1107/S1399004715001388. Epub 2015 Mar 26. PMID:25849394 doi:http://dx.doi.org/10.1107/S1399004715001388
- ↑ Michalska K, Fernandes H, Sikorski M, Jaskolski M. Crystal structure of Hyp-1, a St. John's wort protein implicated in the biosynthesis of hypericin. J Struct Biol. 2010 Feb;169(2):161-71. Epub 2009 Oct 21. PMID:19853038 doi:10.1016/j.jsb.2009.10.008
- ↑ Gasymov OK, Glasgow BJ. ANS fluorescence: potential to augment the identification of the external binding sites of proteins. Biochim Biophys Acta. 2007 Mar;1774(3):403-11. doi: 10.1016/j.bbapap.2007.01.002., Epub 2007 Jan 31. PMID:17321809 doi:http://dx.doi.org/10.1016/j.bbapap.2007.01.002
- ↑ Sliwiak J, Sikorski M, Jaskolski M. PR-10 proteins as potential mediators of melatonin-cytokinin cross-talk in plants: crystallographic studies of LlPR-10.2B isoform from yellow lupine. FEBS J. 2018 Apr 6. doi: 10.1111/febs.14455. PMID:29630775 doi:http://dx.doi.org/10.1111/febs.14455
- ↑ Smaalen, S. van (2007). Incommensurate Crystallography, Oxford University Press
- ↑ Porta J, Lovelace JJ, Schreurs AM, Kroon-Batenburg LM, Borgstahl GE. Processing incommensurately modulated protein diffraction data with Eval15. Acta Crystallogr D Biol Crystallogr. 2011 Jul;67(Pt 7):628-38. doi:, 10.1107/S0907444911017884. Epub 2011 Jun 16. PMID:21697601 doi:http://dx.doi.org/10.1107/S0907444911017884