User:Valentina Dutton/Sandbox 1
From Proteopedia
< User:Valentina Dutton(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
The vast majority of mitochondrial proteins have an N-terminal sequence that indicates that they must be exported to the mitochondria. This sequence is called the mitochondrial signal peptide. When the protein enters the mitochondria, this sequence is usually cleaved, providing greater stability. It is still unknown what this sequence would be in the case of PGAM5 since the protein signal peptide is not cleaved<ref name="sie">PMID:35921890</ref> and PGAM5 is anchored in its entirety to the inner membrane through its transmembrane domain, defined by amino acids 9-29<ref name="cha">PMID:28648608</ref>. | The vast majority of mitochondrial proteins have an N-terminal sequence that indicates that they must be exported to the mitochondria. This sequence is called the mitochondrial signal peptide. When the protein enters the mitochondria, this sequence is usually cleaved, providing greater stability. It is still unknown what this sequence would be in the case of PGAM5 since the protein signal peptide is not cleaved<ref name="sie">PMID:35921890</ref> and PGAM5 is anchored in its entirety to the inner membrane through its transmembrane domain, defined by amino acids 9-29<ref name="cha">PMID:28648608</ref>. | ||
| - | Regarding the catalytic activity, <scene name='96/969636/His105/3'>histidine | + | Regarding the catalytic activity, <scene name='96/969636/His105/3'>histidine 105</scene> is responsible for the nucleophilic attack of the phosphate of the target protein, performing the intermediate link between the protein and the phosphate. However, histidine 105 is part of the canonical RHGE motif, present in most proteins of the PGAM family, forming part of the PGAM domain (98-289)<ref name="cha" />. Protein <scene name='96/969636/Pgam5_dodecamer/2'>oligomerization</scene> is induced by the <scene name='96/969636/Wdxnwd/2'>WDXNWD motif</scene> at amino acids 58-63 which has been shown to function as an allosteric regulator of the specific phosphatase activity of PGAM5<ref name="wil" />. Mutations in this motif prevent oligomerization of the enzyme but still present as <scene name='96/969636/Pgam5_dimer/4'>dimers</scene> since the <scene name='96/969636/Dimerization_motif/2'>C-terminal tail</scene> (270-289) in PGAM5 is responsible for the dimerization of the protein<ref name="cha" />. These dimers, however, do not show phosphatase activity<ref name="wil" />. |
== Function == | == Function == | ||
Even though <scene name='96/969636/Pgam5_monomere/4'>PGAM5</scene> is a member of the PGAM protein family, it appears to lack phosphoglycerate mutase typical phosphotransferase and/or phosphohydrolase activities.<ref name="cha" /> Instead, this protein is a serine/threonine (Ser/Thr) phosphatase, that is, it’s responsible for protein-protein interactions through dephosphorylation of serine/threonine and, occasionally, histidine residues<ref name="che">PMID:33370650</ref><ref name="shi">PMID:19879837</ref>. Its active site is composed of a histidine residue (His-105) responsible for the nucleophilic attack of the phosphorus atom acting as a phospho-acceptor<ref name="cha" /><ref name="shi" />. | Even though <scene name='96/969636/Pgam5_monomere/4'>PGAM5</scene> is a member of the PGAM protein family, it appears to lack phosphoglycerate mutase typical phosphotransferase and/or phosphohydrolase activities.<ref name="cha" /> Instead, this protein is a serine/threonine (Ser/Thr) phosphatase, that is, it’s responsible for protein-protein interactions through dephosphorylation of serine/threonine and, occasionally, histidine residues<ref name="che">PMID:33370650</ref><ref name="shi">PMID:19879837</ref>. Its active site is composed of a histidine residue (His-105) responsible for the nucleophilic attack of the phosphorus atom acting as a phospho-acceptor<ref name="cha" /><ref name="shi" />. | ||
| Line 20: | Line 20: | ||
For a long while, PGAM5’s subcellular localization in the mitochondria has been debated. It includes a transmembrane N-terminal domain, which indicates that it would be located in the IMM, facing the intermembrane space<ref name="bab" />. Nonetheless, it has been demonstrated that PGAM5 interacts with cytosolic proteins, indicating that it could not be located in the IMM, and should be, instead, located in the OMM, facing the outside of the mitochondria<ref name="foo" />. More recently, however, studies have shown that the entire protein is exported to the mitochondria, where it’s anchored in the IMM and, during the first stages of mitophagy, it’s cleaved by the PARL protease in the serine 24 residue and it’s then released in the cytosol, where it interacts with other proteins important to this process<ref name="sie" />. | For a long while, PGAM5’s subcellular localization in the mitochondria has been debated. It includes a transmembrane N-terminal domain, which indicates that it would be located in the IMM, facing the intermembrane space<ref name="bab" />. Nonetheless, it has been demonstrated that PGAM5 interacts with cytosolic proteins, indicating that it could not be located in the IMM, and should be, instead, located in the OMM, facing the outside of the mitochondria<ref name="foo" />. More recently, however, studies have shown that the entire protein is exported to the mitochondria, where it’s anchored in the IMM and, during the first stages of mitophagy, it’s cleaved by the PARL protease in the serine 24 residue and it’s then released in the cytosol, where it interacts with other proteins important to this process<ref name="sie" />. | ||
| - | == Evolutionary | + | == Evolutionary Conservation == |
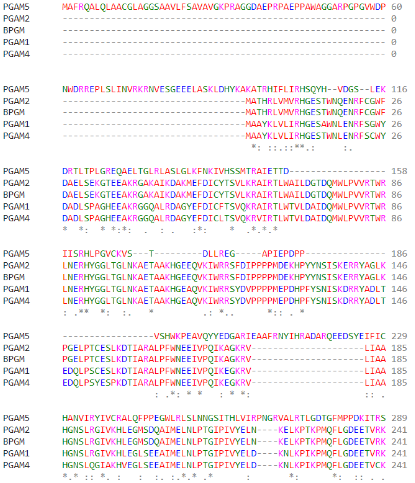
As part of the PGAM protein family, it is expected that PGAM5’s sequence includes a histidine phosphatase superfamily domain typical to this set of proteins, ranging from amino acids 58 up to 287. | As part of the PGAM protein family, it is expected that PGAM5’s sequence includes a histidine phosphatase superfamily domain typical to this set of proteins, ranging from amino acids 58 up to 287. | ||
| Line 26: | Line 26: | ||
[[Image:Familia.png]] | [[Image:Familia.png]] | ||
| - | (Alignment of different PGAM superfamily members) | + | (Clustal Omega Multiple Alignment of different PGAM superfamily members) |
---- | ---- | ||
| Line 33: | Line 33: | ||
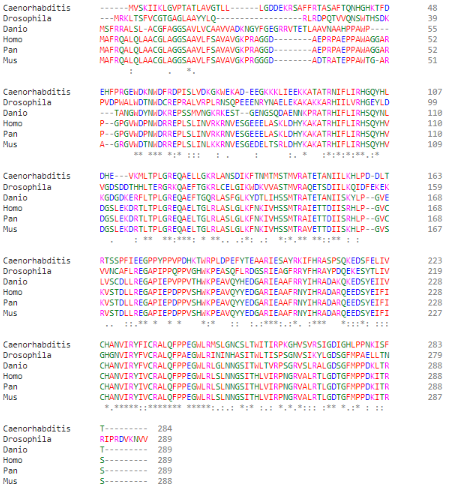
[[Image:Especies.png]] | [[Image:Especies.png]] | ||
| - | (Alignment of PGAM5 in different species, them being: (top to bottom) ''C. elegans; D. melanogaster; D. rerio; H. sapiens; P. paniscus; M. musculus'') | + | (Clustal Omega Multiple Alignment of PGAM5 in different species, them being: (top to bottom) ''C. elegans; D. melanogaster; D. rerio; H. sapiens; P. paniscus; M. musculus'') |
---- | ---- | ||
Current revision
Human Phosphoglycerate Mutase Family Member 5 (PGAM5)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Takeda K, Komuro Y, Hayakawa T, Oguchi H, Ishida Y, Murakami S, Noguchi T, Kinoshita H, Sekine Y, Iemura S, Natsume T, Ichijo H. Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12301-5. Epub 2009 Jul 9. PMID:19590015 doi:http://dx.doi.org/0901823106
- ↑ 2.0 2.1 2.2 Wilkins JM, McConnell C, Tipton PA, Hannink M. A conserved motif mediates both multimer formation and allosteric activation of phosphoglycerate mutase 5. J Biol Chem. 2014 Sep 5;289(36):25137-48. PMID:25012655 doi:10.1074/jbc.M114.565549
- ↑ 3.0 3.1 Siebert V, Silber M, Heuten E, Muhle-Goll C, Lemberg MK. Cleavage of mitochondrial homeostasis regulator PGAM5 by the intramembrane protease PARL is governed by transmembrane helix dynamics and oligomeric state. J Biol Chem. 2022 Jul 31:102321. doi: 10.1016/j.jbc.2022.102321. PMID:35921890 doi:http://dx.doi.org/10.1016/j.jbc.2022.102321
- ↑ 4.0 4.1 4.2 4.3 4.4 Chaikuad A, Filippakopoulos P, Marcsisin SR, Picaud S, Schroder M, Sekine S, Ichijo H, Engen JR, Takeda K, Knapp S. Structures of PGAM5 Provide Insight into Active Site Plasticity and Multimeric Assembly. Structure. 2017 Jul 5;25(7):1089-1099.e3. doi: 10.1016/j.str.2017.05.020. Epub, 2017 Jun 22. PMID:28648608 doi:http://dx.doi.org/10.1016/j.str.2017.05.020
- ↑ Cheng M, Lin N, Dong D, Ma J, Su J, Sun L. PGAM5: A crucial role in mitochondrial dynamics and programmed cell death. Eur J Cell Biol. 2021 Jan;100(1):151144. PMID:33370650 doi:10.1016/j.ejcb.2020.151144
- ↑ 6.0 6.1 Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009 Oct 30;139(3):468-84. PMID:19879837 doi:10.1016/j.cell.2009.10.006
- ↑ 7.0 7.1 Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006 Dec 8;281(49):37893-903. PMID:17046835 doi:10.1074/jbc.M606539200
- ↑ Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res. 2008 May 1;314(8):1789-803. doi: 10.1016/j.yexcr.2008.02.014. Epub , 2008 Mar 5. PMID:18387606 doi:http://dx.doi.org/10.1016/j.yexcr.2008.02.014
- ↑ 9.0 9.1 9.2 Baba T, Tanimura S, Yamaguchi A, Horikawa K, Yokozeki M, Hachiya S, Iemura SI, Natsume T, Matsuda N, Takeda K. Cleaved PGAM5 dephosphorylates nuclear serine/arginine-rich proteins during mitophagy. Biochim Biophys Acta Mol Cell Res. 2021 Jun;1868(7):119045. PMID:33872670 doi:10.1016/j.bbamcr.2021.119045
- ↑ 10.0 10.1 Liang MZ, Ke TL, Chen L. Mitochondrial Protein PGAM5 Emerges as a New Regulator in Neurological Diseases. Front Mol Neurosci. 2021 Sep 23;14:730604. PMID:34630036 doi:10.3389/fnmol.2021.730604
- ↑ 11.0 11.1 11.2 Lu W, Karuppagounder SS, Springer DA, Allen MD, Zheng L, Chao B, Zhang Y, Dawson VL, Dawson TM, Lenardo M. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson's-like movement disorder. Nat Commun. 2014 Sep 15;5:4930. PMID:25222142 doi:10.1038/ncomms5930
- ↑ Ng Kee Kwong F, Nicholson AG, Pavlidis S, Adcock IM, Chung KF. PGAM5 expression and macrophage signatures in non-small cell lung cancer associated with chronic obstructive pulmonary disease (COPD). BMC Cancer. 2018 Dec 10;18(1):1238. PMID:30526542 doi:10.1186/s12885-018-5140-9
- ↑ He GW, Günther C, Kremer AE, Thonn V, Amann K, Poremba C, Neurath MF, Wirtz S, Becker C. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury. Gut. 2017 Apr;66(4):716-723. PMID:27566130 doi:10.1136/gutjnl-2015-311247
- ↑ Li CJ, Lin LT, Tsai HW, Wen ZH, Tsui KH. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell. 2022 Feb;21(2):e13546. PMID:34995407 doi:10.1111/acel.13546