Avelox (moxifloxacin)
From Proteopedia
| Line 1: | Line 1: | ||
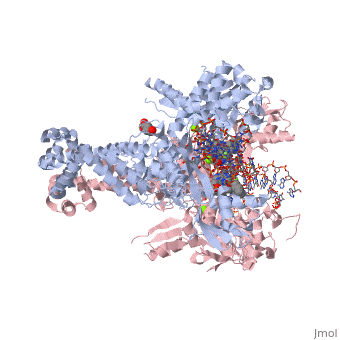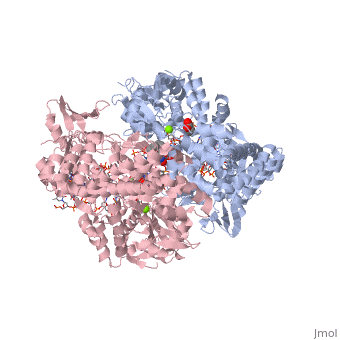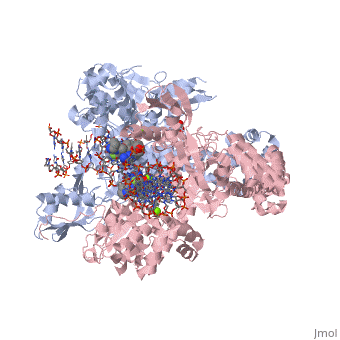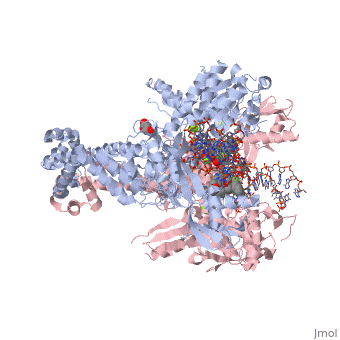
<StructureSection load='2xkk' size='340' side='right' caption='Crystal structure of Moxifloxacin, DNA and a Baumannii Topo IV' scene=''> | <StructureSection load='2xkk' size='340' side='right' caption='Crystal structure of Moxifloxacin, DNA and a Baumannii Topo IV' scene=''> | ||
== Structure == | == Structure == | ||
| - | Moxifloxacin is a synthetic antimicrobial fluoroquinolone with the molecular formula C<sub>21</sub>H<sub>24</sub>FN<sub>3</sub>O<sub>4</sub>. It has an average molecular weight of 401.438 g/mol '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. Fluoroquinolones are organic compounds categorized as a quinoline, aromatic ring with a substituted carboxyl group at one or more positions, as well as a fluoride as a central part of the compound '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. The compound is able to accept eight hydrogen bonds and donate two '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The structure of the drug can also be found as a form of a monohydrochloride salt '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. | + | <scene name='75/756549/Moxifloxacin/1'>Moxifloxacin</scene> is a synthetic antimicrobial fluoroquinolone with the molecular formula C<sub>21</sub>H<sub>24</sub>FN<sub>3</sub>O<sub>4</sub>. It has an average molecular weight of 401.438 g/mol '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. Fluoroquinolones are organic compounds categorized as a quinoline, aromatic ring with a substituted carboxyl group at one or more positions, as well as a fluoride as a central part of the compound '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. The compound is able to accept eight hydrogen bonds and donate two '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The structure of the drug can also be found as a form of a monohydrochloride salt '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. |
| - | + | ||
| - | [[Image:Moxi 3d.png]]<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref> | ||
== Function == | == Function == | ||
Moxifloxacin is a fluoroquinolone antibiotic that also acts as an antibacterial compound '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The drug is typically taken in 400mg tablets daily, ranging from five to fourteen days. When dealing with more severe infections, moxifloxacin is administered in 400mg through intravenous injections '''<ref> https://livertox.nlm.nih.gov//Moxifloxacin.htm </ref>'''. Moxifloxacin is active against gram-positive and gram-negative bacteria with an increased affinity for gram-positive bacteria, such as the multidrug-resistant strains of ''Streptococcus pneumoniae'' '''<ref> https://livertox.nlm.nih.gov//Moxifloxacin.htm </ref>'''. Other examples of infections for which moxifloxacin is prescribed include bronchitis, sinusitis, skin or soft tissue infection, anthrax prophylaxis, and tuberculosis '''<ref> https://www.drugs.com/avelox.html</ref>'''. The function of moxifloxacin is inhibited by the consumption of cations, but the precise mechanism is unknown. One possible explanation for the decreased function of moxifloxacin in the presence of cations is the binding of cations to the compound causing chelation and results in a decreased amount of drug available to interact with the bacteria. Alternatively, the presence of cations may interact with the drug’s target, DNA <scene name='75/756549/Gyrase/1'>gyrase</scene>, which decreases its effectiveness '''<ref> https://www.ncbi.nlm.nih.gov/pubmed/7868402 </ref>'''. | Moxifloxacin is a fluoroquinolone antibiotic that also acts as an antibacterial compound '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The drug is typically taken in 400mg tablets daily, ranging from five to fourteen days. When dealing with more severe infections, moxifloxacin is administered in 400mg through intravenous injections '''<ref> https://livertox.nlm.nih.gov//Moxifloxacin.htm </ref>'''. Moxifloxacin is active against gram-positive and gram-negative bacteria with an increased affinity for gram-positive bacteria, such as the multidrug-resistant strains of ''Streptococcus pneumoniae'' '''<ref> https://livertox.nlm.nih.gov//Moxifloxacin.htm </ref>'''. Other examples of infections for which moxifloxacin is prescribed include bronchitis, sinusitis, skin or soft tissue infection, anthrax prophylaxis, and tuberculosis '''<ref> https://www.drugs.com/avelox.html</ref>'''. The function of moxifloxacin is inhibited by the consumption of cations, but the precise mechanism is unknown. One possible explanation for the decreased function of moxifloxacin in the presence of cations is the binding of cations to the compound causing chelation and results in a decreased amount of drug available to interact with the bacteria. Alternatively, the presence of cations may interact with the drug’s target, DNA <scene name='75/756549/Gyrase/1'>gyrase</scene>, which decreases its effectiveness '''<ref> https://www.ncbi.nlm.nih.gov/pubmed/7868402 </ref>'''. | ||
Revision as of 15:41, 18 April 2017
| |||||||||||
References
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://livertox.nlm.nih.gov//Moxifloxacin.htm
- ↑ https://livertox.nlm.nih.gov//Moxifloxacin.htm
- ↑ https://www.drugs.com/avelox.html
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/7868402
- ↑ https://www.drugbank.ca/drugs/DB00218
- ↑ https://www.ncbi.nlm.nih.gov/books/NBK21703/
- ↑ https://academic.oup.com/nar/article/44/10/4528/2516939/How-topoisomerase-IV-can-efficiently-unknot-and
- ↑ http://aac.asm.org/content/43/1/12.full
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696358/
- ↑ http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html
- ↑ http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=Chemical-and-Physical-Properties
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://www.drugs.com/avelox.html
(1)https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=Chemical-and-Physical-Properties (2)https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug (3)https://livertox.nlm.nih.gov//Moxifloxacin.htm (4)https://www.drugs.com/avelox.html (5)https://www.ncbi.nlm.nih.gov/pubmed/7868402 6. https://www.drugbank.ca/drugs/DB00218 7. https://www.ncbi.nlm.nih.gov/books/NBK21703/ 8.https://academic.oup.com/nar/article/44/10/4528/2516939/How-topoisomerase-IV-can-efficiently-unknot-and 9. http://aac.asm.org/content/43/1/12.full 10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696358/ 11.http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html Photographs :Ginsburg AS, Hooper N, Parrish N, Dooley KE, Dorman SE, Booth J, Diener-West M, Merz WG, Bishai WR, Sterling TR: Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis. 2003 Dec 1;37(11):1448-52. Epub 2003 Nov 4.