Avelox (moxifloxacin)
From Proteopedia
| Line 1: | Line 1: | ||
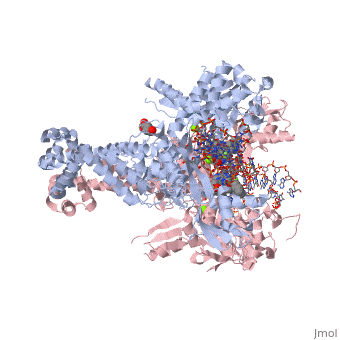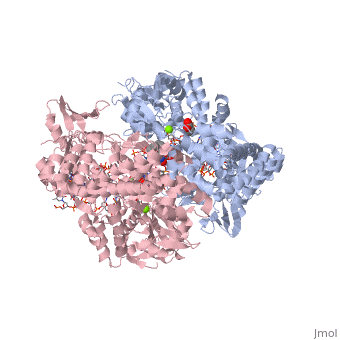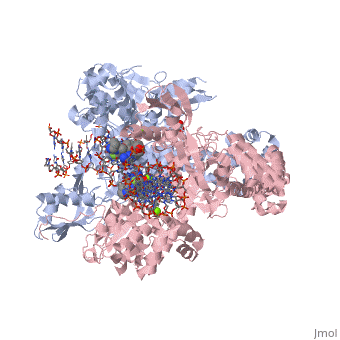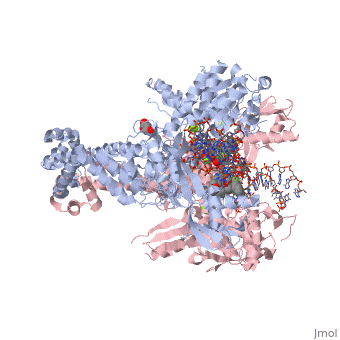
<StructureSection load='2xkk' size='340' side='right' caption='Crystal structure of Moxifloxacin, DNA and a Baumannii Topo IV' scene=''> | <StructureSection load='2xkk' size='340' side='right' caption='Crystal structure of Moxifloxacin, DNA and a Baumannii Topo IV' scene=''> | ||
== Structure == | == Structure == | ||
| - | <scene name='75/756549/Moxifloxacin/1'>Moxifloxacin</scene> is a synthetic antimicrobial fluoroquinolone with the molecular formula C<sub>21</sub>H<sub>24</sub>FN<sub>3</sub>O<sub>4</sub>. It has an average molecular weight of 401.438 g/mol '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. Fluoroquinolones are organic compounds categorized as a quinoline, aromatic ring with a substituted carboxyl group at one or more positions, as well as a fluoride as a central part of the compound '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. The compound is able to accept eight hydrogen bonds and donate two '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The structure of the drug can also be found as a form of a monohydrochloride salt '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. | + | <scene name='75/756549/Moxifloxacin/1'>Moxifloxacin</scene> is a synthetic antimicrobial fluoroquinolone with the molecular formula C<sub>21</sub>H<sub>24</sub>FN<sub>3</sub>O<sub>4</sub>. It has an average molecular weight of 401.438 g/mol '''<ref name="pubchem"> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. Fluoroquinolones are organic compounds categorized as a quinoline, aromatic ring with a substituted carboxyl group at one or more positions, as well as a fluoride as a central part of the compound '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. The compound is able to accept eight hydrogen bonds and donate two '''<ref> https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure </ref>'''. The structure of the drug can also be found as a form of a monohydrochloride salt '''<ref> https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug</ref>'''. |
== Function == | == Function == | ||
Revision as of 15:57, 18 April 2017
| |||||||||||
References
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=2D-Structure
- ↑ https://livertox.nlm.nih.gov//Moxifloxacin.htm
- ↑ https://livertox.nlm.nih.gov//Moxifloxacin.htm
- ↑ https://www.drugs.com/avelox.html
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/7868402
- ↑ https://www.drugbank.ca/drugs/DB00218
- ↑ https://www.ncbi.nlm.nih.gov/books/NBK21703/
- ↑ https://academic.oup.com/nar/article/44/10/4528/2516939/How-topoisomerase-IV-can-efficiently-unknot-and
- ↑ http://aac.asm.org/content/43/1/12.full
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696358/
- ↑ http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html
- ↑ http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=Chemical-and-Physical-Properties
- ↑ https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug
- ↑ https://www.drugs.com/avelox.html
(1)https://pubchem.ncbi.nlm.nih.gov/compound/moxifloxacin#section=Chemical-and-Physical-Properties (2)https://drugsdetails.com/moxifloxacin/#Pharmacophore_structure_Information_about_the_chemical_structure_of_the_drug (3)https://livertox.nlm.nih.gov//Moxifloxacin.htm (4)https://www.drugs.com/avelox.html (5)https://www.ncbi.nlm.nih.gov/pubmed/7868402 6. https://www.drugbank.ca/drugs/DB00218 7. https://www.ncbi.nlm.nih.gov/books/NBK21703/ 8.https://academic.oup.com/nar/article/44/10/4528/2516939/How-topoisomerase-IV-can-efficiently-unknot-and 9. http://aac.asm.org/content/43/1/12.full 10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696358/ 11.http://vanderbilt.edu/vicb/DiscoveriesArchives/combatting_antibiotic_drug_resistance.html Photographs :Ginsburg AS, Hooper N, Parrish N, Dooley KE, Dorman SE, Booth J, Diener-West M, Merz WG, Bishai WR, Sterling TR: Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis. 2003 Dec 1;37(11):1448-52. Epub 2003 Nov 4.