Journal:Acta Cryst F:S2053230X20010122
From Proteopedia
(Difference between revisions)

| Line 9: | Line 9: | ||
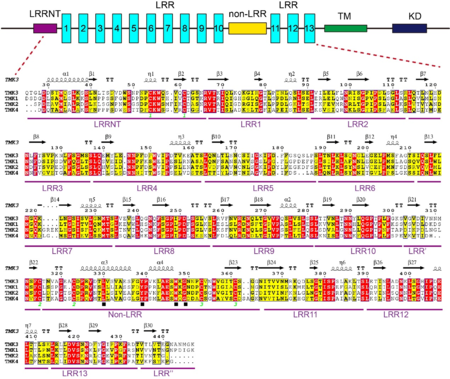
A block of about 40 residues termed non-LRR region intersects the LRR domain into two subdomains – a N-terminal LRR subdomain with 10 LRRs (N-LRRs) and a C-terminal LRR subdomain with 3 LRRs (C-LRRs). C-LRR packs nearly perpendicularly against the spine of N-LRR. Most LRRs from the N-LRR contain the sequence GxL/i/vP (x stands for any amino acid) specific for the plant LRR proteins, with the only exceptions for LRR3, LRR4, LRR7 and LRR10 (see static image below). | A block of about 40 residues termed non-LRR region intersects the LRR domain into two subdomains – a N-terminal LRR subdomain with 10 LRRs (N-LRRs) and a C-terminal LRR subdomain with 3 LRRs (C-LRRs). C-LRR packs nearly perpendicularly against the spine of N-LRR. Most LRRs from the N-LRR contain the sequence GxL/i/vP (x stands for any amino acid) specific for the plant LRR proteins, with the only exceptions for LRR3, LRR4, LRR7 and LRR10 (see static image below). | ||
| - | [[Image: | + | [[Image:Tabg.png|thumb|350px|left|Sequence alignment of LRRs in TMK3. The boundary of each LRR is shown on its right. The conserved residues are shown with yellow background. The solid black star indicates the amino acids specific to plant LRR proteins.]] |
{{Clear}} | {{Clear}} | ||
The non-LRR has a Cys cluster with the pattern of ''Cx''<sub>''6-7''</sub>''Cx''<sub>''29-30''</sub>''Cx''<sub>''6-11''</sub>''C'' (Cys315-Cys323 and Cys353-Cys361) and a conserved motif of Lx8Yx7-8WxG (Figure 2) similar to Yx8KG found in many LRR- receptor-like kinases (Fritz-Laylin ''et al.,'' 2005<ref name="Fritz">PMID:15955925</ref>). | The non-LRR has a Cys cluster with the pattern of ''Cx''<sub>''6-7''</sub>''Cx''<sub>''29-30''</sub>''Cx''<sub>''6-11''</sub>''C'' (Cys315-Cys323 and Cys353-Cys361) and a conserved motif of Lx8Yx7-8WxG (Figure 2) similar to Yx8KG found in many LRR- receptor-like kinases (Fritz-Laylin ''et al.,'' 2005<ref name="Fritz">PMID:15955925</ref>). | ||
| - | [[Image:Qw.png|thumb| | + | [[Image:Qw.png|thumb|450px|left|]] |
{{Clear}} | {{Clear}} | ||
Most of LRR structures have caps, which shield the hydrophobic core of the first LRR unit at the N-terminus and the last unit at the C-terminus. In extracellular proteins or extracellular regions, the N-terminal and C-terminal caps frequently consist of Cys clusters including two or four Cys residues. The Cys clusters on the N- terminal and C-terminal sides of the LRR arcs are called LRRNT and LRRCT, respectively. Almost all known typical LRR structures include an LRRNT, some of which also have an LRRCT. TMK3-LRR belongs to the LRR subgroups that lacks LRRCT and possesses only an LRRNT (with Cx6C) characterized by a disulfide bond between Cys54 and Cys61. We have also observed five asparagine residues (N165, N170, N223, N286 and N448) modified by N-glycosylation (Figure 3C). | Most of LRR structures have caps, which shield the hydrophobic core of the first LRR unit at the N-terminus and the last unit at the C-terminus. In extracellular proteins or extracellular regions, the N-terminal and C-terminal caps frequently consist of Cys clusters including two or four Cys residues. The Cys clusters on the N- terminal and C-terminal sides of the LRR arcs are called LRRNT and LRRCT, respectively. Almost all known typical LRR structures include an LRRNT, some of which also have an LRRCT. TMK3-LRR belongs to the LRR subgroups that lacks LRRCT and possesses only an LRRNT (with Cx6C) characterized by a disulfide bond between Cys54 and Cys61. We have also observed five asparagine residues (N165, N170, N223, N286 and N448) modified by N-glycosylation (Figure 3C). | ||
Revision as of 13:27, 4 August 2020
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.