User:Isabela de Aquino Zogbi/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==Introduction== | ==Introduction== | ||
| - | <scene name='91/915204/Dysferlin/2'>Dysferlin</scene> is a large transmembrane protein of approximately 230kDa encoded by the dysferlin gene (DYSF omim) highly expressed in striated skeletal and cardiac muscle, but can be found in kidney, placenta, lung and brain tissues. Dysferlin is a protein that belongs to the same family of genes as ''Caenorhabditis elegans'' ferlin, also known as ferlin-like proteins, therefore the name it was given, and can also be known as ferlin 1-like 1 (Fer1L1). It is common to this family the presence of type II transmembrane domains, where the most part of the protein faces de cytoplasm <ref name="omim"> OMIM: https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin; https://www.sciencedirect.com/science/article/pii/S0955067407000993</ref>. This protein is critical for repair of muscle membranes after damage and its mutation lead to a progressive muscle dystrophy, since in its absence the membrane tear is not adequately repaired leading to myofiber necrosis and gradual and progressive loss of muscle tissue <ref> https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013854 </ref> <ref name="ref5"/>. The protein rapidly responds to injury with a calcium (Ca2+) influx mechanism which aids the repair. Dysferlin-deficient muscle fibers demonstrate a primary defect in Ca2+-dependent vesicle-mediated membrane repair <ref name="ref5"> https://www.sciencedirect.com/science/article/pii/S0022283619301883 </ref>. | + | <scene name='91/915204/Dysferlin/2'>Dysferlin</scene> is a large transmembrane protein of approximately 230kDa encoded by the dysferlin gene (DYSF omim) highly expressed in striated skeletal and cardiac muscle, but can be found in kidney, placenta, lung and brain tissues. Dysferlin is a protein that belongs to the same family of genes as ''Caenorhabditis elegans'' ferlin, also known as ferlin-like proteins, therefore the name it was given, and can also be known as ferlin 1-like 1 (Fer1L1). It is common to this family the presence of type II transmembrane domains, where the most part of the protein faces de cytoplasm <ref name="omim"> OMIM: https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin; https://www.sciencedirect.com/science/article/pii/S0955067407000993</ref>. This protein is critical for repair of muscle membranes after damage and its mutation lead to a progressive muscle dystrophy, since in its absence the membrane tear is not adequately repaired leading to myofiber necrosis and gradual and progressive loss of muscle tissue <ref name="ref1"> https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013854 </ref> <ref name="ref5"/>. The protein rapidly responds to injury with a calcium (Ca2+) influx mechanism which aids the repair. Dysferlin-deficient muscle fibers demonstrate a primary defect in Ca2+-dependent vesicle-mediated membrane repair <ref name="ref5"> https://www.sciencedirect.com/science/article/pii/S0022283619301883 </ref>. |
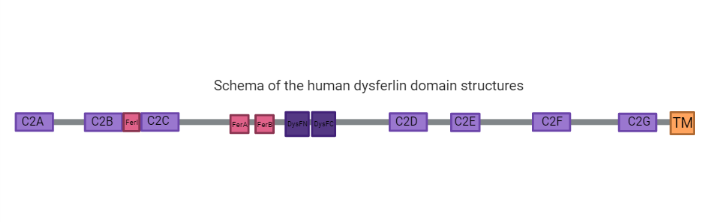
== Structure and Function == | == Structure and Function == | ||
| Line 29: | Line 29: | ||
====C2 Domains function==== | ====C2 Domains function==== | ||
| - | C2 domains are calcium sensitive phospholipid binding domains with an approximate length of 130 amino acids <ref name="ref5"/>, while the function of the Dysf domain remains unclear <ref | + | C2 domains are calcium sensitive phospholipid binding domains with an approximate length of 130 amino acids <ref name="ref5"/>, while the function of the Dysf domain remains unclear <ref name="ref1"/> (2). The presence of the C2 domains is common to ferlin-like proteins, in which only the C2A domain binds strongly to lipids in a calcium dependent way. The other domains have weaker bonds or are calcium independent. Also, C2A, E and F domains have shown a relevant function in the protein activity <ref name="ref5"/>. In response to elevated calcium concentrations, these domains target a protein to a particular membrane compartment based upon preference for an organelle specific lipid headgroup. After binding, some C2 domains actively cluster lipids or bend the membrane, actively perturbing membrane structure in helping to facilitate cellular processes (8). |
| Line 50: | Line 50: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | [3] https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin; https://www.sciencedirect.com/science/article/pii/S0955067407000993 | ||
Revision as of 16:10, 19 June 2022
Dysferlin
| |||||||||||
References
- ↑ 1.0 1.1 OMIM: https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin; https://www.sciencedirect.com/science/article/pii/S0955067407000993
- ↑ 2.0 2.1 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013854
- ↑ 3.0 3.1 3.2 3.3 3.4 https://www.sciencedirect.com/science/article/pii/S0022283619301883
- ↑ https://link.springer.com/article/10.1186/1472-6807-14-3
- ↑ 5.0 5.1 https://onlinelibrary.wiley.com/doi/full/10.1111/j.1600-0854.2011.01267.x
- ↑ https://www.sciencedirect.com/science/article/pii/S0005273614000108?via%3Dihub