Carbon monoxide dehydrogenase
From Proteopedia
| Line 1: | Line 1: | ||
{{STRUCTURE_3b52| PDB=3b52 | SIZE=500| SCENE=|right|CAPTION=Carbon monoxide dehydrogenase with Fe4S4, Fe2S2, Fe3NiS4 clusters complex with Fe and CO2, [[3b52]] }} | {{STRUCTURE_3b52| PDB=3b52 | SIZE=500| SCENE=|right|CAPTION=Carbon monoxide dehydrogenase with Fe4S4, Fe2S2, Fe3NiS4 clusters complex with Fe and CO2, [[3b52]] }} | ||
| + | <!-- | ||
| + | Please use the "3D" button above this box to insert a Jmol applet (molecule) on this page. | ||
| + | Or use the four-green-boxes-button to insert scrollable text adjacent | ||
| + | to a Jmol applet. Check out the other buttons as well! | ||
| + | --> | ||
'''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see [[Acetyl-CoA synthase]]). | '''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see [[Acetyl-CoA synthase]]). | ||
Revision as of 11:52, 9 May 2012
Template:STRUCTURE 3b52 Carbon monoxide dehydrogenase (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see Acetyl-CoA synthase).
N-Butylisocyanide Oxidation at the [NiFe4S4OHx]-cluster of CO Dehydrogenase
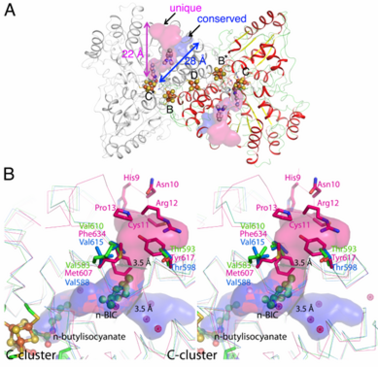
[1] Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO2 as their sole carbon and/or energy source. The with ~ 130 kDa containing , called B, B’, C, C’ and D. Each subunit contains the and cubane-type [Fe4S4] . Another [Fe4S4] connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe4S4OHx] (CO + H2O ↔ CO2 + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H2 and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster.
The high resolution crystal structure of CODH-II from Carboxydothermus hydrogenoformans (dmin = 1.28 Å) revealed (the product of n-BIC oxidation), in which the (2yiv) rather than the (3b52) and states (3i39). , while . A superposition of the CODH-II structures with different ligands bound to the C-cluster reveals a flexible coordination and geometry for the Ni-Fe1 dyad, while the [Fe3S4] moiety of C-cluster remains unchanged in position.
, which was shown to be a binding place for Xe atoms in CODH/ACS (2z8y). Analysis of the CODH-II structure identified the presence of two different channels (see below), in which one is analogous to the channel identified by Xe-soaking in bifunctional CODH/ACS (conserved channel), while the other seems to be specific for monofunctional CODH (unique channel). The conserved channel in CODH-II is similar in position to that in bifunctional CODH, where the channel is extended to reach the A-cluster of ACS. Monofunctional CODHs have smaller side chains like Thr and Val, while Tyr617 and Phe634 block a passage of the unique channel to the protein surface in bifunctional CODH/ACS. Furthermore, the unique channel is completely blocked by residue His9 – Pro13 to prevent diffusion of gaseous substrate/product from the protein in bifunctional CODH/ACS. However, the channel of monofunctional CODH-II is directed towards the solvent, which is in line with its role to allow fast progress and egress of substrates and products from the active site to the outside of the enzyme, which has not been described previously.
3D structures of carbon monoxide dehydrogenase
CODH monofunctional
1ffu – HpCODH + Fe2S2 + FAD – Hydrogenophaga pseudoflava
1ffv - HpCODH + Fe2S2 + molybdenum cofactor + FAD
1jqk - CODH + Fe4S4 + Fe + Fe3-Ni-S4 – Rhodospirillum rubrum
1n60 - OcCODH + Fe2S2 + H2MoO3 + pterin cytosine dinucleotide + FAD – Oligotropha carboxidovorans
1n61, 1n62, 1n63, 1n5w, 1zxi - OcCODH + Fe2S2 + Cu-Mo cluster + pterin cytosine dinucleotide + FAD
1su6, 1su7, 1su8, 1suf - ChCODH + Fe4S4 + Fe2S2 + Fe4-Ni-S5 – Carboxydothermus hydrogenoformans
3b51, 3b53 - ChCODH + Fe4S4 + Fe2S2 + Fe + Fe3-Ni
3b52 - ChCODH + Fe4S4 + Fe2S2 + Fe + CO2 + Fe3-Ni-S4
3i39 - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + CN + Fe3-Ni-S4
2yiv - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + butyl-isocyanide + Fe3-Ni-S4
CODH/ACS bifunctional
1mjg, 1oao, 2z8y, 3i01 – MtCODH/ACS α+β + Fe4-Ni-S4 – Moorella thermoacetica
3i04 - MtCODH/ACS α+β + CN + Fe4-Ni-S4
3git - MtCODH/ACS α + Fe4S4
3s2x - MtCODH/ACS α C terminal + Ni
2h9a - ChCODH/ACS γ + Fe4S4
2ycl - ChCODH/ACS γ + Fe4S4 + cobalamin
- ↑ Jeoung JH, Dobbek H. n-Butyl isocyanide oxidation at the [NiFe(4)S (4)OH ( x )] cluster of CO dehydrogenase. J Biol Inorg Chem. 2011 Sep 9. PMID:21904889 doi:10.1007/s00775-011-0839-y
