Carbon monoxide dehydrogenase
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='CODH2-nBIC-Dimer1.pdb' size='450' side='right' scene='Journal:JBIC:13/Cv/5' caption=''> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
'''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see [[Acetyl-CoA synthase]]). | '''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see [[Acetyl-CoA synthase]]). | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
| - | <br /> | ||
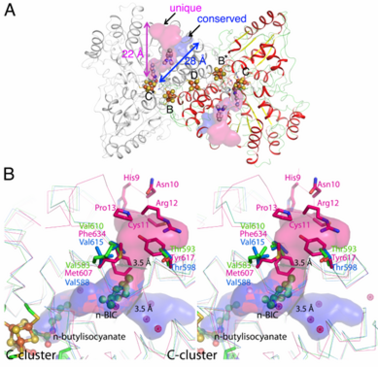
==={{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of Carbon monoxide dehydrogenase=== | ==={{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of Carbon monoxide dehydrogenase=== | ||
Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. | Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. | ||
| - | + | ||
The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | ||
Revision as of 08:54, 28 August 2013
| |||||||||||
3D structures of carbon monoxide dehydrogenase
Updated on 28-August-2013
CODH monofunctional
1ffu – HpCODH + Fe2S2 + FAD – Hydrogenophaga pseudoflava
1ffv - HpCODH + Fe2S2 + molybdenum cofactor + FAD
1jqk - CODH + Fe4S4 + Fe + Fe3-Ni-S4 – Rhodospirillum rubrum
1n60 - OcCODH + Fe2S2 + H2MoO3 + pterin cytosine dinucleotide + FAD – Oligotropha carboxidovorans
1n61, 1n62, 1n63, 1n5w, 1zxi - OcCODH + Fe2S2 + Cu-Mo cluster + pterin cytosine dinucleotide + FAD
1su6, 1su7, 1su8, 1suf - ChCODH + Fe4S4 + Fe2S2 + Fe4-Ni-S5 – Carboxydothermus hydrogenoformans
3b51, 3b53 - ChCODH + Fe4S4 + Fe2S2 + Fe + Fe3-Ni
3b52 - ChCODH + Fe4S4 + Fe2S2 + Fe + CO2 + Fe3-Ni-S4
3i39 - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + CN + Fe3-Ni-S4
2yiv - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + butyl-isocyanide + Fe3-Ni-S4
CODH/ACS bifunctional
1mjg, 1oao, 2z8y, 3i01 – MtCODH/ACS α+β + Fe4-Ni-S4 – Moorella thermoacetica
3i04 - MtCODH/ACS α+β + CN + Fe4-Ni-S4
3git - MtCODH/ACS α + Fe4S4
3s2x - MtCODH/ACS α C terminal + Ni
2h9a - ChCODH/ACS γ + Fe4S4
2ycl - ChCODH/ACS γ + Fe4S4 + cobalamin
- ↑ Jeoung JH, Dobbek H. n-Butyl isocyanide oxidation at the [NiFe(4)S (4)OH ( x )] cluster of CO dehydrogenase. J Biol Inorg Chem. 2011 Sep 9. PMID:21904889 doi:10.1007/s00775-011-0839-y