Odorant binding protein
From Proteopedia
| Line 37: | Line 37: | ||
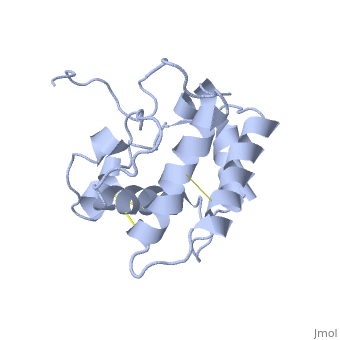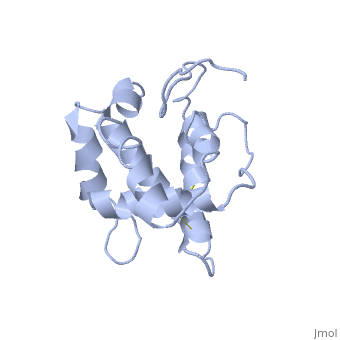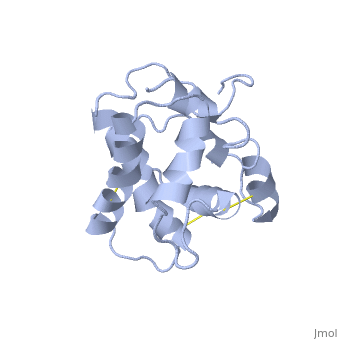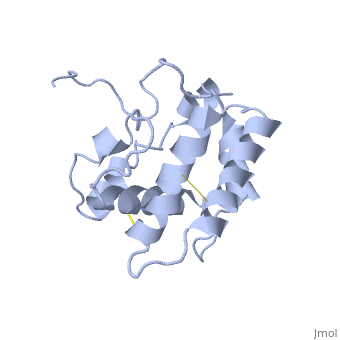
The protein has 164 amino acids that forms 6-7 alpha helices. Three disulfide bonds (in yellow) formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust sturcture of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_residues/1'>charged residues</scene>, which allows it to make interactions with the water molecule and sollubilize in the sensillar lymph. | The protein has 164 amino acids that forms 6-7 alpha helices. Three disulfide bonds (in yellow) formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust sturcture of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_residues/1'>charged residues</scene>, which allows it to make interactions with the water molecule and sollubilize in the sensillar lymph. | ||
| - | + | ====BmorPBP ligand and ligand binding==== | |
The protein natural ligand is the moth pheromone <scene name='68/683383/Bombykol_ligand_in_2p71/1'>Bombykol</scene>. However, it was demonstrated that other molecules can also bound to the protein cavity <ref>doi: 10.1016/j.str.2007.07.013</ref>. The interaction with the ligand is beeing made by 4 alpha helices 1, 4, 5 and 6 in the core of the protein, which form the binding cavity <ref>doi: 10.1016/S1074-5521(00)00078-8</ref>. | The protein natural ligand is the moth pheromone <scene name='68/683383/Bombykol_ligand_in_2p71/1'>Bombykol</scene>. However, it was demonstrated that other molecules can also bound to the protein cavity <ref>doi: 10.1016/j.str.2007.07.013</ref>. The interaction with the ligand is beeing made by 4 alpha helices 1, 4, 5 and 6 in the core of the protein, which form the binding cavity <ref>doi: 10.1016/S1074-5521(00)00078-8</ref>. | ||
Inside the binding cavity, <scene name='68/683383/Residues_interacting/1'>non-charged residues</scene> are interacting with the pheromone, mainly by van der waals bounds. Out of those residues, some are conserved across OBP of lepidopteran (<font color='green'><b>in green</b></font>), and the rest are conserved in lepidopteran PBP only (<font color='light blue'><b>in light blue</b></font>). | Inside the binding cavity, <scene name='68/683383/Residues_interacting/1'>non-charged residues</scene> are interacting with the pheromone, mainly by van der waals bounds. Out of those residues, some are conserved across OBP of lepidopteran (<font color='green'><b>in green</b></font>), and the rest are conserved in lepidopteran PBP only (<font color='light blue'><b>in light blue</b></font>). | ||
In addition, the hydroxyl group of the pheromone bombykol forms a <scene name='68/683383/Ser56_interaction_with_oxg/1'>hydrogen bond with the sidechain of Ser56</scene>, Ser56 in red, oxygens are in purple (O–O distance of 2.8 Å). | In addition, the hydroxyl group of the pheromone bombykol forms a <scene name='68/683383/Ser56_interaction_with_oxg/1'>hydrogen bond with the sidechain of Ser56</scene>, Ser56 in red, oxygens are in purple (O–O distance of 2.8 Å). | ||
| - | + | ====Protein conformations==== | |
| - | + | BmorPBP has two conformations, The "open form" (A) and the "close form" (B)<ref>DOI: 10.1074/jbc.274.43.30950</ref>. The bombykol and the alpha-helix loacated in the C terminus of the protein compete for the binding site: when the c-terminus is inside the binding cavity it forms an alpha helix, and the protien is in its "close form" (B), wherease in the "open form" (A) the c terminus is outside of the protein and has no defined secondery shpae. | |
| - | + | ||
| - | + | ||
| - | The "open form" (A) and the "close form" (B)<ref>DOI: 10.1074/jbc.274.43.30950</ref>. | + | |
| - | The | + | |
In a nuetral pH (6.5-7) the protein is in the "open form" (A), in which the | In a nuetral pH (6.5-7) the protein is in the "open form" (A), in which the | ||
| Line 54: | Line 50: | ||
The C terminus of the protein bears mostly [[nonpolar amino acids]]. Yet on the surface of the helix there are three exceptional amino acids: Asp-132, Glu-137, and Glu-141, which are conserved in moth PBP <ref>doi: 10.1016/j.bbrc.2005.07.176</ref>. Of these, residues [[Asp-132 and Glu-141]] triggers the formation of the α-helix upon protonation at low pH. This what is causing the ejacullate of the ligand from the binding pocket, which is replaced by the formated alpha helix<ref>doi: 10.1016/j.bbrc</ref>. | The C terminus of the protein bears mostly [[nonpolar amino acids]]. Yet on the surface of the helix there are three exceptional amino acids: Asp-132, Glu-137, and Glu-141, which are conserved in moth PBP <ref>doi: 10.1016/j.bbrc.2005.07.176</ref>. Of these, residues [[Asp-132 and Glu-141]] triggers the formation of the α-helix upon protonation at low pH. This what is causing the ejacullate of the ligand from the binding pocket, which is replaced by the formated alpha helix<ref>doi: 10.1016/j.bbrc</ref>. | ||
| - | + | The <scene name='68/683383/1dqe-1gm0/2'>transition between the two conforamtion</scene> is both pH and ligand dependent <ref>doi: 10.1073?pnas.251532998</ref><ref>DOI: 10.1016/j.bbrc.2005.07.176</ref><ref>doi: 10.1073/pnas.1317706110</ref>. | |
| - | + | ||
| - | <scene name='68/683383/1dqe-1gm0/2'>conformation changing</scene> | ||
{{Button Toggle AnimationOnPause}} | {{Button Toggle AnimationOnPause}} | ||
Revision as of 10:26, 11 January 2015
Contents |
Introduction
Odorant-binding protein (OBP) are soluble proteins which involve in the processes of odorant detection in the olfactory sensilla.
The first OBP that was identified is Bovine odorant binding protein, that was isolated from a cow's mucus ref. Though functunaly same, vertebrates and insects OBP have different origin and stucture. OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly Drosophila melanogaster is required for the detection of the pheromone vaccenyl acetate [Ha and Smith, 2006; Xu et al., 2005] and has been proven to adopt a conformation that activates the odorant receptor [Laughlin et al., 2008].
OBP in insects
OBP Function
Despite five decades of intensive research, the exact roles of OBP and the mechanism by which the odorant receptor (OR) is activated are still in dispute [1][2].
A few functions have been suggested for OBP: 1. Solubelizing the odorant molecule and its transportation in the sensillar lymph.
2. Protecting the odorant molecule from the odorant degrading enzymes, in the sensillar lymph.
3. Activating of the odorant receptor on the dendrite membrane, by the odorant-OBP complex.
4. Mediating the deactivation of the odorant molecule after the activation of the receptor.
5. An organic anion (the protein has 9 negative charges).
Of all, the first role of OBP as an odorant solubilizer and carrier is generally accepted.
In order to explain the structure and function of these fascinating proteins, this page will further focus on a particular OBP - the well investigated Bombyx mori PBP: BmorPBP.
Bombyx mori BmorPBP (lets talk about sex..)
| |||||||||||
See also
References
Proteopedia Page Contributors and Editors (what is this?)
Nurit Eliash, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky