Sandbox myosinkinesin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ==Kinesin== | |
| - | + | <StructureSection load='4uxr' size='340' side='right' caption='This is the head region of kinesin' scene=''> | |
| - | == | + | |
| - | <StructureSection load='4uxr' size='340' side='right' caption=' | + | |
This is a default text for your page '''Sandbox myosinkinesin'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page '''Sandbox myosinkinesin'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| Line 17: | Line 15: | ||
== Structural highlights == | == Structural highlights == | ||
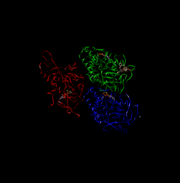
| - | [[Image:131631646354632.jpg]] | + | [[Image:131631646354632.jpg]] This is the overall structure of a functional kinesin dimer. |
| + | |||
| + | |||
| + | '''''Heavy Chain''''' | ||
| + | |||
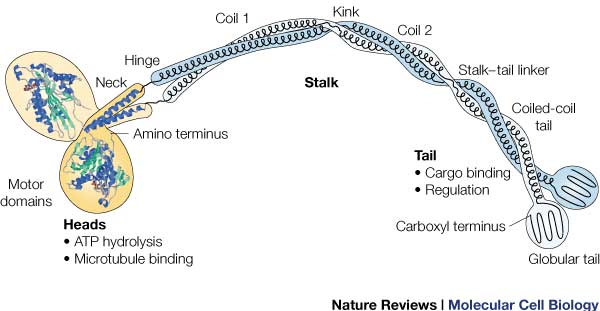
| + | It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure. | ||
| + | |||
| + | |||
| + | '''''Light Chain''''' | ||
| + | Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. | ||
| + | |||
'''Head''' | '''Head''' | ||
| + | Head: Most conserved domain amongst all kinesin, it consists mainly of alpha helixes. Its tertiary structure usually includes a large cleft from the actin binding site to the ATP binding pocket. The head has the ability to bind microtubule on one site and bind ATP at another. This section undergoes the most conformational change and is responsible for the force that causes kinesin to move along the cytoskeleton. In kinesin, binding of ATP appears to have allosteric control over the binding of kinesin to the tubules, by a twisting of a β- sheet core, showing allosteric control between the subdomains within the protein. | ||
'''Neck''' | '''Neck''' | ||
Revision as of 22:24, 15 December 2015
Kinesin
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644