Carboxypeptidase A
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
=====S1 Subsite===== | =====S1 Subsite===== | ||
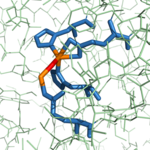
In the same way that the S1' subsite is involved in anchoring the polypeptide substrate in place, the <scene name='69/694222/3cpas1subsitespacefill/2'>S1 subsite</scene> (spacefill view, subsite in cyan) contains several residues that help hold the substrate in the active site, but the S1 subsite also contains the residues that are involved in the catalytic chemical mechanism. In general, the residues of the <scene name='69/694222/3cpas1subsitemeshfill/1'>S1 subsite</scene> (pseudo-mesh view, subsite in cyan) have polar or charged side chains that either allow for hydrogen bonding to stabilize negatively charged intermediates of the hydrolysis reaction or position particular atoms appropriately to allow for chemistry to occur. Three residues (<scene name='69/694222/3cpas1subsiteresidues1/1'>Asn144, Arg145, and Tyr248</scene>) aid in the recognition of the C-terminal residue of a polypeptide substrate.<ref name="CPA1" /> The Asn144 and Tyr248 residues each engage in [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] and [http://en.wikipedia.org/wiki/Intermolecular_force ion-dipole interactions] with the carboxyl group at the C-terminus, while Arg145 provides additional stability by participating in [http://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/ ion-ion interactions] with the carboxyl group (see Figure 2 in the section titled "Mechanism of Action"). <scene name='69/694222/3cpas1subsiteresidues2/1'>Arg71</scene> helps stabilize the substrate in the active site by engaging in ion-dipole interactions with the carbonyl oxygen of the penultimate substrate residue (Figure 2). Three residues (<scene name='69/694222/3cpas1subsitezn/1'>His196, Glu72, and His69</scene>) are liganded to a catalytic Zn<sup>2+</sup> ion that is complexed to a water molecule positioned one bond distance away from the C-terminal peptide bond carbonyl carbon (Figure 2).<ref name="CPA2" /> <scene name='69/694222/3cpas1subsiteglu270/1'>Glu270</scene> deprotonates this water molecule and acts as a base catalyst in the hydrolysis mechanism (Figure 2). <scene name='69/694222/3cpas1subsitearg127/1'>Arg127</scene>, along with the positively charged Zn<sup>2+</sup> ion, help stabilize the negatively charged intermediate generated in the [http://www.masterorganicchemistry.com/tips/addition-elimination/ addition-elimination] step of the hydrolysis reaction (Figure 2).<ref name="CPA2" /> | In the same way that the S1' subsite is involved in anchoring the polypeptide substrate in place, the <scene name='69/694222/3cpas1subsitespacefill/2'>S1 subsite</scene> (spacefill view, subsite in cyan) contains several residues that help hold the substrate in the active site, but the S1 subsite also contains the residues that are involved in the catalytic chemical mechanism. In general, the residues of the <scene name='69/694222/3cpas1subsitemeshfill/1'>S1 subsite</scene> (pseudo-mesh view, subsite in cyan) have polar or charged side chains that either allow for hydrogen bonding to stabilize negatively charged intermediates of the hydrolysis reaction or position particular atoms appropriately to allow for chemistry to occur. Three residues (<scene name='69/694222/3cpas1subsiteresidues1/1'>Asn144, Arg145, and Tyr248</scene>) aid in the recognition of the C-terminal residue of a polypeptide substrate.<ref name="CPA1" /> The Asn144 and Tyr248 residues each engage in [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] and [http://en.wikipedia.org/wiki/Intermolecular_force ion-dipole interactions] with the carboxyl group at the C-terminus, while Arg145 provides additional stability by participating in [http://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/ ion-ion interactions] with the carboxyl group (see Figure 2 in the section titled "Mechanism of Action"). <scene name='69/694222/3cpas1subsiteresidues2/1'>Arg71</scene> helps stabilize the substrate in the active site by engaging in ion-dipole interactions with the carbonyl oxygen of the penultimate substrate residue (Figure 2). Three residues (<scene name='69/694222/3cpas1subsitezn/1'>His196, Glu72, and His69</scene>) are liganded to a catalytic Zn<sup>2+</sup> ion that is complexed to a water molecule positioned one bond distance away from the C-terminal peptide bond carbonyl carbon (Figure 2).<ref name="CPA2" /> <scene name='69/694222/3cpas1subsiteglu270/1'>Glu270</scene> deprotonates this water molecule and acts as a base catalyst in the hydrolysis mechanism (Figure 2). <scene name='69/694222/3cpas1subsitearg127/1'>Arg127</scene>, along with the positively charged Zn<sup>2+</sup> ion, help stabilize the negatively charged intermediate generated in the [http://www.masterorganicchemistry.com/tips/addition-elimination/ addition-elimination] step of the hydrolysis reaction (Figure 2).<ref name="CPA2" /> | ||
| + | |||
| + | ==Important Tyr248 Residue== | ||
| + | 1CPX’s most interesting distinction from other CPA proteins is that its crystallographic data revealed a different conformation of the Tyr248 residue (CITE main article)(SHOW PICTURE). Previous literature has suggested that the conserved Tyr residue amongst CPA proteins has been involved in an induced fit mechanism (BLUE LINK) because it typically has been found pointing outward toward solution (GREEN LINK) when a substrate is not bound to the enzyme (see 3CPA (BLUE LINK)) (CITE main article). However, 1CPX's crystallographic data shows Tyr248 pointing toward the active site (GREEN LINK) without a substrate bound. Therefore, this denies the previously proposed induced fit mechanism for CPA proteins and suggests that Tyr 248 is a ligand to the catalytic Zn (SUPERSCRIPT) in 1CPX (CITE MAIN ARTICLE). Moreover, this data supports that this is the native conformation of Tyr248 in solution because none of the residues in the protein’s active site interact in the crystallographic packing (main article, 59, 60). Not only does Tyr248 point toward the active site, but in doing so, Tyr248 caps the hydrophobic binding pocket (GREEN LINK) with its interactions with Ile247 and a hydrogen bond (cite). | ||
=====Putting It All Together===== | =====Putting It All Together===== | ||
Revision as of 19:31, 2 April 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Carboxypeptidase A in Bos taurus
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Bukrinsky JT, Bjerrum MJ, Kadziola A. 1998. Native carboxypeptidase A in a new crystal environment reveals a different conformation of the important tyrosine 248. Biochemistry. 37(47):16555-16564. DOI: 10.1021/bi981678i
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Christianson DW, Lipscomb WN. 1989. Carboxypeptidase A. Acc. Chem. Res. 22:62-69.
- ↑ Suh J, Cho W, Chung S. 1985. Carboxypeptidase A-catalyzed hydrolysis of α-(acylamino)cinnamoyl derivatives of L-β-phenyllactate and L-phenylalaninate: evidence for acyl-enzyme intermediates. J. Am. Chem. Soc. 107:4530-4535. DOI: 10.1021/ja00301a025
- ↑ Geoghegan, KF, Galdes, A, Martinelli, RA, Holmquist, B, Auld, DS, Vallee, BL. 1983. Cryospectroscopy of intermediates in the mechanism of carboxypeptidase A. Biochem. 22(9):2255-2262. DOI: 10.1021/bi00278a031
- ↑ Kaplan, AP, Bartlett, PA. 1991. Synthesis and evaluation of an inhibitor of carboxypeptidase A with a Ki value in the femtomolar range. Biochem. 30(33):8165-8170. PMID: 1868091
- ↑ Worthington, K., Worthington, V. 1993. Worthington Enzyme Manual: Enzymes and Related Biochemicals. Freehold (NJ): Worthington Biochemical Corporation; [2011; accessed March 28, 2017]. Carboxypeptidase A. http://www.worthington-biochem.com/COA/
- ↑ Pitout, MJ, Nel, W. 1969. The inhibitory effect of ochratoxin a on bovine carboxypeptidase a in vitro. Biochem. Pharma. 18(8):1837-1843. DOI: 0.1016/0006-2952(69)90279-2
- ↑ Normant, E, Martres, MP, Schwartz, JC, Gros, C. 1995. Purification, cDNA cloning, functional expression, and characterization of a 26-kDa endogenous mammalian carboxypeptidase inhibitor. Proc. Natl. Acad. Sci. 92(26):12225-12229. PMCID: PMC40329
Student Contributors
- Thomas Baldwin
- Michael Melbardis
- Clay Schnell
Proteopedia Page Contributors and Editors (what is this?)
Michael Melbardis, Douglas Schnell, Thomas Baldwin, Geoffrey C. Hoops, Michal Harel