User:Sunghwan Cho/Sandbox reserved 899
From Proteopedia
| Line 27: | Line 27: | ||
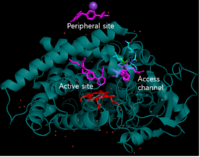
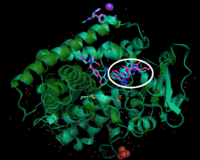
Moreover, '''Figure 4''' didn't show any conformational changes between CYP2C9 WT and CYP2C9*30. '''Figure 5''' represents how CYP2C9 binds to losartan. In the experiment, 1:10 dilution applied and losartan binds to three binding sites: peripheral site, active site, access channel. when SNP induces CYP2C9 single amino acid change, the alanine (Ala) at the position of 477 changes into polar threonine. This amino acid change leads binding difference between 2C9WT and 2C9*30. '''Figure 6''' showed that there is losartan reoriented in *30 comparing to WT. | Moreover, '''Figure 4''' didn't show any conformational changes between CYP2C9 WT and CYP2C9*30. '''Figure 5''' represents how CYP2C9 binds to losartan. In the experiment, 1:10 dilution applied and losartan binds to three binding sites: peripheral site, active site, access channel. when SNP induces CYP2C9 single amino acid change, the alanine (Ala) at the position of 477 changes into polar threonine. This amino acid change leads binding difference between 2C9WT and 2C9*30. '''Figure 6''' showed that there is losartan reoriented in *30 comparing to WT. | ||
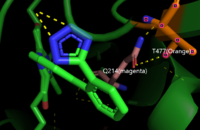
| - | CYP2C9 WT doesn’t show side-chain interaction from Glutamine 214. However, the variant *30 shows the interaction between Glutamine 214(Q214) and Threonine(477) and this leads to disturbance on *30 interaction with Losartan in the access channel('''Figure 7'''). | + | CYP2C9 WT doesn’t show side-chain interaction from Glutamine 214. However, the variant *30 shows the interaction between Glutamine 214(Q214) and Threonine(477) and this leads to a disturbance on *30 interaction with Losartan in the access channel('''Figure 7'''). |
| - | [[Image:CYP2C9WT_with_Losartan.png|thumb|200px|left|Figure 5.CYP2C9 Wild Type with losartan binding. Cyan: protein, orange:heme, magenta:Losartan]] | + | [[Image:CYP2C9WT_with_Losartan.png|thumb|200px|left|Figure 5.CYP2C9 Wild Type with losartan binding. Cyan: protein, orange: heme, magenta: Losartan]] |
| - | [[Image:CYP2C9WT_vs_star30.png|thumb|200px|right|Figure 6.The overlay of CYP2C9WT and CYP2C9*30. This figure shows the binding difference between CYP2C9 WT and the CYP2C9*30 variant. The circled region is where Losartan | + | [[Image:CYP2C9WT_vs_star30.png|thumb|200px|right|Figure 6.The overlay of CYP2C9WT and CYP2C9*30. This figure shows the binding difference between CYP2C9 WT and the CYP2C9*30 variant. The circled region is where Losartan binds differently in WT comparing to *30 variant. the Green: *30variant, cyan: WT.]] |
[[Image:CYP2C9_star30.png|thumb|200px|left|Figure 7. CYP2C9*30 side-chain interaction between Q214 and T477.]] | [[Image:CYP2C9_star30.png|thumb|200px|left|Figure 7. CYP2C9*30 side-chain interaction between Q214 and T477.]] | ||
| - | [[Image:Losartan_within_active_binding_site_of_CYP2C9star30.png]] | + | [[Image:Losartan_within_active_binding_site_of_CYP2C9star30.png|thumb|200px|center|Figure 8.Losartan binding to the active site of CYP2C9*30]] |
== Pharmacogenomics of Cytochrome P450 == | == Pharmacogenomics of Cytochrome P450 == | ||
Pharmacogenomics is the study of the genetic basis of variation in drug response. While people with standard genetic bases have a normal response to drug administration, some people could have different drug responses because of their genetic variation. Polymorphism is the genetic variation on more than 1% of the population, whereas less than 1% of the population is mutation. For example, CYP2D6 is a good example of polymorphism. CYP2D6 has three different types of polymorphism: Poor metabolizer(PM), Extensive metabolizer(WT, standard), Ultrarapid metabolizer(URM). For PM of CYP2D6, CYP2D6*5 leads poor metabolism on drug input because they lose their major region of CYP2D6 genes. For URM of CYP2D6, CYP2D6*2XN has multiple copies of CYP2D6 genes, and this leads to metabolizing much faster than the standard one (WT). | Pharmacogenomics is the study of the genetic basis of variation in drug response. While people with standard genetic bases have a normal response to drug administration, some people could have different drug responses because of their genetic variation. Polymorphism is the genetic variation on more than 1% of the population, whereas less than 1% of the population is mutation. For example, CYP2D6 is a good example of polymorphism. CYP2D6 has three different types of polymorphism: Poor metabolizer(PM), Extensive metabolizer(WT, standard), Ultrarapid metabolizer(URM). For PM of CYP2D6, CYP2D6*5 leads poor metabolism on drug input because they lose their major region of CYP2D6 genes. For URM of CYP2D6, CYP2D6*2XN has multiple copies of CYP2D6 genes, and this leads to metabolizing much faster than the standard one (WT). | ||
| - | Polymorphism is separated into two categories: synonomous SNP and nonsynonomous SNP. Synonomous SNP is when one nucleotide of codon changed, there is no change in amino acid, hence no drug response changes. However, nonsynonomous SNP induces amino acid sequence change and responds differently, as mentioned in CYP2D6.2.<ref>Brunton, L., Knollmann, B., & Hilal-Dandan, R. (2018). Goodman & Gilman's | + | Polymorphism is separated into two categories: synonomous SNP and nonsynonomous SNP. Synonomous SNP is when one nucleotide of codon changed, there is no change in amino acid, hence no drug response changes. However, nonsynonomous SNP induces amino acid sequence change and responds differently, as mentioned in CYP2D6.2.<ref>Brunton, L., Knollmann, B., & Hilal-Dandan, R. (2018). Goodman & Gilman's |
| - | Pharmacological | + | The Pharmacological Basis of Therapeutics. New York, N.Y.: McGraw-Hill Education LLC.</ref> |
Even for CYP2C9, there are many types of variation on drug input. For example, CYP2C9*2(Arg144Cys) and CYP2C9*3(Ile359Leu) have reduced enzyme activity on warfarin. Reduced enzyme activity means a person who has this type of variant would have very sensitive to warfarin. Therefore, patients should be recommended with lower does.<ref>Laura, D., 2016. Warfarin Therapy and the Genotypes CYP2C9 and VKORC1. Medical Genetics Summaries,.</ref> | Even for CYP2C9, there are many types of variation on drug input. For example, CYP2C9*2(Arg144Cys) and CYP2C9*3(Ile359Leu) have reduced enzyme activity on warfarin. Reduced enzyme activity means a person who has this type of variant would have very sensitive to warfarin. Therefore, patients should be recommended with lower does.<ref>Laura, D., 2016. Warfarin Therapy and the Genotypes CYP2C9 and VKORC1. Medical Genetics Summaries,.</ref> | ||
Revision as of 17:39, 24 April 2020
|
Contents |
Introduction to Cytochrome P450 2C9 and CYP2C9*30
Cytochrome P450(CYP) is a monooxygenase and heme-containing enzyme found in the endoplasmic reticulum(ER) membrane. CYPs have the function of the biogenesis of sterols and hormones. Also, it involves in the detoxification of foreign chemicals and the metabolism of drugs. The alphabet "P" stands for the pigment from liver microsomes, and the wavelength at 450nm is measured when heme iron in CYP is reduced and interacts with carbon monoxide [1]. The major function of CYPs is to interact with a clinically available drug, including nonsteroidal anti-inflammatory drug (NASID) ibuprofen, antihypertensive losartan, and the anticoagulant warfarin; especially CYP3A4, CYP2D6, and CYP2C9 are enzymes that involve phase Ⅰ metabolism. CYPs have different family, sub-family, and the individual members of sub-family that provide a different function. For example, CYP2C9 is 2 for family, C for sub-family, and 9 for individual members of sub-family.
Furthermore, Single nucleotide polymorphism(SNP) renders more diversification rather than wild type classification. SNP is a variation in a single base pair in a DNA sequence that may lead to the variation in the amino acid of the protein. Such variations may influence the function of CYPs that leads to a negative effect on an individual's response, such as drug-drug interactions. [2], [3]. The general schematic representation of the CYP represented in Figure 1.
The Metabolism of Cytochrome P450
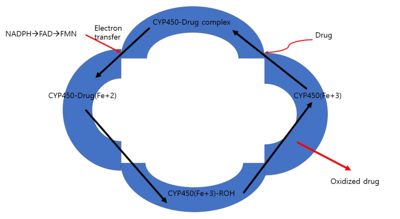
CYPs associate with cytochrome P450 oxidoreductase (CPR) and cytochrome b5 for catalysis of a substrate. These two coenzymes serve as electrons transport. CPR is composed of NADPH/FAD-binding domain, linker domain, FMN (flavin mononucleotide) binding domain, and N-terminal membrane domain.
CPR has the “closed-open transition” conformation. When there are no electrons available, CPR is closed with only the presence of the interaction with FAD. However, when there is NADPH presence near NADPH binding domain, CPR induces open conformation for electron transport onto CYS. When CPR binds to FMN via FMN binding domain, NADPH or FADH2 will transfer electrons FMN and to the heme group of CYPs, which gives rise to oxidation of substrates, as electrons transfer from the FMN of CPR to the heme of CYP.[6]
When Open conformation of CPR interact with CYP, the binding interaction between the positively charged basic proximal surface of CYP and the negatively charged FMN and FAD/NADPH domains of CPR. Also, this interaction is mainly based on electrostatic interaction.[7] Furthermore, Site-directed mutagenesis on some amino acid sequences proceeded to see whether the turnover rate of interaction between CYP and CPR could change. Those amino acid sequences could have the likelihood of binding site of CPR for CYP. Mutants had a much lower turnover rate than the wild type. The amino acid sequences including Lys 94, Lys 99, Lys 105, Lys 440, Lys 453, Arg 455, Lys 463, Arg clusters Arg 135-Arg 136-Arg 137 are significant residues for ionic bond for CYP-CPR interaction.[8] Furthermore, even for lysine and arginine from CYP have an important role in the interaction with CPR. For example, CYP4502B1 has lysines at 251, 384, 422, 433, and 473. These residues are going to induce electrostatic interaction and lead to make a complex of CYP-CPR.[9]
The structural importance in Cytochrome and Comparative analysis between CYP2C9 Wild type and CYP2C9*30
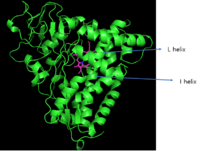
The structural feature of cytochrome P450 is a tertiary structure that is composed of a mixture of α-helix and β-sheet connected by loops. Figure 3 is CYP P450cam, which is first discovered CYP P450 and they have certain significant regions that share most CYPs. As the secondary elements close to the heme group, it is likely to have conserved sequences; I and L helix are conserved sequences.[10] Furthermore, the heme group of CYP2C9 is stabilized by hydrogen bonds between the propionates group and side chains of Trp120, Arg124, His368, and Arg433. Arg 97 also forms a hydrogen bond to propionates as well as carbonyl oxygen group of Val113 and Pro367.[11]

Moreover, Figure 4 didn't show any conformational changes between CYP2C9 WT and CYP2C9*30. Figure 5 represents how CYP2C9 binds to losartan. In the experiment, 1:10 dilution applied and losartan binds to three binding sites: peripheral site, active site, access channel. when SNP induces CYP2C9 single amino acid change, the alanine (Ala) at the position of 477 changes into polar threonine. This amino acid change leads binding difference between 2C9WT and 2C9*30. Figure 6 showed that there is losartan reoriented in *30 comparing to WT.
CYP2C9 WT doesn’t show side-chain interaction from Glutamine 214. However, the variant *30 shows the interaction between Glutamine 214(Q214) and Threonine(477) and this leads to a disturbance on *30 interaction with Losartan in the access channel(Figure 7).
Pharmacogenomics of Cytochrome P450
Pharmacogenomics is the study of the genetic basis of variation in drug response. While people with standard genetic bases have a normal response to drug administration, some people could have different drug responses because of their genetic variation. Polymorphism is the genetic variation on more than 1% of the population, whereas less than 1% of the population is mutation. For example, CYP2D6 is a good example of polymorphism. CYP2D6 has three different types of polymorphism: Poor metabolizer(PM), Extensive metabolizer(WT, standard), Ultrarapid metabolizer(URM). For PM of CYP2D6, CYP2D6*5 leads poor metabolism on drug input because they lose their major region of CYP2D6 genes. For URM of CYP2D6, CYP2D6*2XN has multiple copies of CYP2D6 genes, and this leads to metabolizing much faster than the standard one (WT). Polymorphism is separated into two categories: synonomous SNP and nonsynonomous SNP. Synonomous SNP is when one nucleotide of codon changed, there is no change in amino acid, hence no drug response changes. However, nonsynonomous SNP induces amino acid sequence change and responds differently, as mentioned in CYP2D6.2.[13]
Even for CYP2C9, there are many types of variation on drug input. For example, CYP2C9*2(Arg144Cys) and CYP2C9*3(Ile359Leu) have reduced enzyme activity on warfarin. Reduced enzyme activity means a person who has this type of variant would have very sensitive to warfarin. Therefore, patients should be recommended with lower does.[14]
Structural highlights
Image:Star30 with losartan.pse
</StructureSection>
References
- ↑ OMURA T, SATO R. A new cytochrome in liver microsomes. J Biol Chem. 1962 Apr;237:1375-6. PMID:14482007
- ↑ Meyer U.A., Zanger U., Skoda R., Grant D.M. (1989) Genetic Polymorphisms of Drug-Metabolizing Enzymes: Molecular Mechanisms. In: Dahl S.G., Gram L.F. (eds) Clinical Pharmacology in Psychiatry. Psychopharmacology Series, vol 7. Springer, Berlin, Heidelberg DOI https://doi.org/10.1007/978-3-642-74430-3_15
- ↑ Daly AK. Pharmacogenetics of the cytochromes P450. Curr Top Med Chem. 2004;4(16):1733-44. doi: 10.2174/1568026043387070. PMID:15579105 doi:http://dx.doi.org/10.2174/1568026043387070
- ↑ Brunton, L., Knollmann, B., & Hilal-Dandan, R. (2018). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York, N.Y.: McGraw-Hill Education LLC ISBN: 978-1-25-958473-2
- ↑ Ghosh, C., Hossain, M., Solanki, J., Dadas, A., Marchi, N., & Janigro, D. (2016). Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discovery Today, 21(10), 1609-1619. doi: 10.1016/j.drudis.2016.06.004
- ↑ Sugishima, M., Sato, H., Higashimoto, Y., Harada, J., Wada, K., Fukuyama, K. and Noguchi, M., 2014. Structural basis for the electron transfer from an open form of NADPH-cytochrome P450 oxidoreductase to heme oxygenase. Proceedings of the National Academy of Sciences, 111(7), pp.2524-2529.
- ↑ Kandel, S. and Lampe, J., 2014. Role of Protein–Protein Interactions in Cytochrome P450-Mediated Drug Metabolism and Toxicity. Chemical Research in Toxicology, 27(9), pp.1474-1486.
- ↑ Toru, S., Tetsumi, T., Masahiro, H. and Yoshiaki, F., 1991. Probing the Role of Lysines and Arginines in the Catalytic Function of Cytochrome P450d by Site-directed Mutagenesis. The Journal OF BIOLOGICAL CHEMISTRY, 266(6), pp.3372-3375.
- ↑ Shen, S. and Strobel, H., 1993. Role of Lysine and Arginine Residues of Cytochrome P450 in the Interaction between Cytochrome P4502B1 and NADPH-Cytochrome P450 Reductase. Archives of Biochemistry and Biophysics, 304(1), pp.257-265.
- ↑ Ortiz de Montellano, P., 2015. Cytochrome P450 Structure, Mechanism, Biochemistry. 4th ed.
- ↑ Williams, P., Cosme, J., Ward, A. et al. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature 424, 464–468 (2003). https://doi.org/10.1038/nature01862
- ↑ Kessel, A., and Ben-Tal, N. (2018). Introduction to proteins_structure, function, and motion. 2nd ed.
- ↑ Brunton, L., Knollmann, B., & Hilal-Dandan, R. (2018). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York, N.Y.: McGraw-Hill Education LLC.
- ↑ Laura, D., 2016. Warfarin Therapy and the Genotypes CYP2C9 and VKORC1. Medical Genetics Summaries,.