User:Eduardo Soares/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
==c-di-GMP== | ==c-di-GMP== | ||
| + | |||
| + | Cyclic dimeric guanosine monophosphate (c-di-GMP) was initially discovered by Benziman and colleagues as an allosteric activator of the membrane-bound cellulose synthase in Gluconacetobacter xylinus. It serves as a soluble molecule functioning as a second messenger in bacteria. Its role encompasses the stimulation of adhesin biosynthesis, exopolysaccharide matrix substance production in biofilms, and the inhibition of various forms of motility. This dynamic regulation controls the transition between planktonic motile behavior and the sedentary lifestyle associated with biofilm formation in bacteria (Figure 1). Furthermore, c-di-GMP governs virulence in animal and plant pathogens, cell cycle progression, antibiotic production, and other cellular functions. However, the precise molecular mechanisms underlying these processes and the specific targets affected by c-di-GMP-binding effector components are still being elucidated. | ||
| + | |||
| + | The synthesis of c-di-GMP involves the conversion of two guanosine triphosphate (GTP) molecules by diguanylate cyclases (DGCs), while its breakdown yields 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) through the action of specific phosphodiesterases (PDEs). pGpG is subsequently cleaved into two guanosine monophosphate (GMP) molecules (Figure 1). DGC activity is associated with the GGDEF domain, named after the crucial amino acid sequence motif found in the enzyme's active site. On the other hand, c-di-GMP-specific PDE activity is linked to the EAL or HD-GYP domains, with their respective amino acid motifs playing essential roles in their enzymatic functions. | ||
| + | |||
| + | The significance of c-di-GMP regulatory systems gained considerable attention following the advent of whole genome sequencing, revealing the widespread presence of GGDEF and EAL domains in bacterial species, often with a large number of encoded proteins. Artificial modulation of cellular c-di-GMP levels through overproduction of GGDEF domain proteins led to enhanced adhesin and biofilm matrix component synthesis, while impairing motility and acute virulence functions. Conversely, overproduction of EAL domain proteins produced opposite phenotypes (Figure 1). Current research aims to assign specific molecular functions to GGDEF, EAL, and HD-GYP domain proteins and unravel their integration into complex regulatory networks. Notably, recent findings have identified biologically active GGDEF and EAL domain proteins lacking DGC or PDE activity, exemplifying their involvement in transcriptional regulation and RNA degradation. | ||
| + | |||
| + | |||
==Molecular Interaction== | ==Molecular Interaction== | ||
Revision as of 13:11, 23 June 2023
VCA0042/plzD complexed with c-di-GMP
Introduction
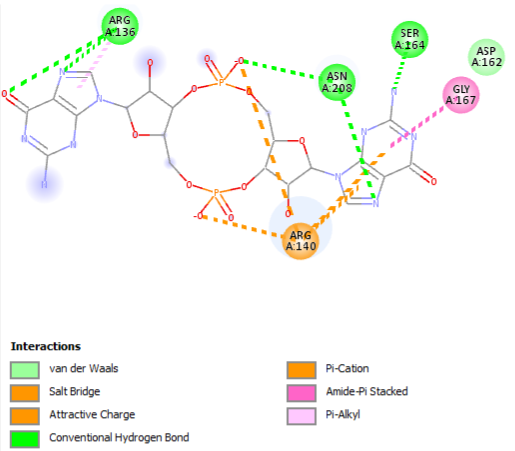
The PDB code 2RDE represents a PilZ protein complexed with cyclic diguanylate monophosphate (c-di-GMP) in Vibrio cholera there is a molecular interaction involved in the regulation of bacterial biofilm formation and motility. The complex is formed by the binding of the small signaling molecule c-di-GMP to a protein domain known as PilZ. This interaction plays a crucial role in bacterial physiology and is implicated in various cellular processes.
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Benach J, Swaminathan SS, Tamayo R, Handelman SK, Folta-Stogniew E, Ramos JE, Forouhar F, Neely H, Seetharaman J, Camilli A, Hunt JF. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 2007 Dec 12;26(24):5153-66. doi: 10.1038/sj.emboj.7601918. Epub 2007 Nov 22. PMID: 18034161; PMCID: PMC2140105.