User:Gustavo Sartorelli de Carvalho Rego/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
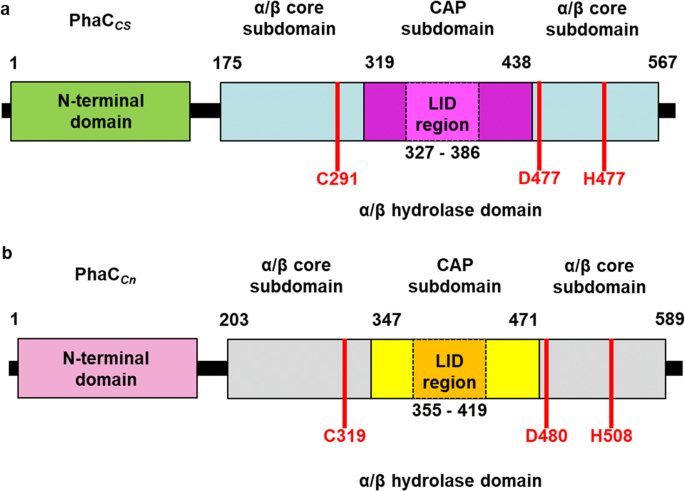
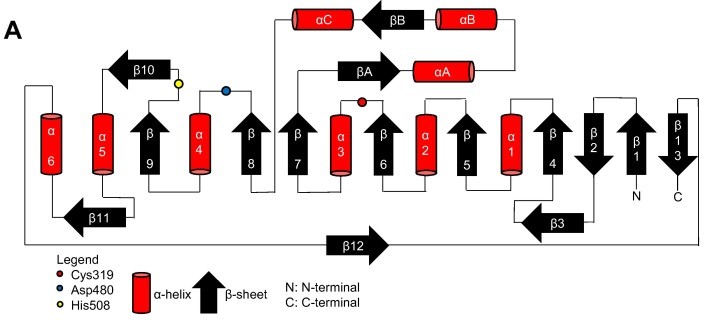
Thanks to the X-ray cristallography data from [https://proteopedia.org/wiki/index.php/5hz2 PhaC<sub>cn</sub>-CAT] and [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT], it was possible to categorize the class 1 phaCs in based on their molecular organization, made of two domains: The N-terminal domain and the <scene name='10/1050300/Phac_i_catalytic_c_domain/1'>catalytic C-terminal domain</scene>. <b><span class='text-gray'>The C-terminal domain</span></b> carries the <scene name='10/1050300/Phac_i_catalytic_site/2'>catalytic site, formed by the aminoacid triad</scene> <b><span class='text-red'>(Cys-Asp-His)</span></b> located deep within the <b><span class='text-gray'>hydrophobic</span></b> <scene name='10/1050300/Phac_i_polar/1'>cavity</scene>, that in the closed conformation is partially covered by the <b><span class='text-orange'>Cap</span></b> <scene name='10/1050300/Phac_i_cap_subdomain/1'>subdomain</scene>.<ref name='Neoh' /><ref name='Chek' /> | Thanks to the X-ray cristallography data from [https://proteopedia.org/wiki/index.php/5hz2 PhaC<sub>cn</sub>-CAT] and [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT], it was possible to categorize the class 1 phaCs in based on their molecular organization, made of two domains: The N-terminal domain and the <scene name='10/1050300/Phac_i_catalytic_c_domain/1'>catalytic C-terminal domain</scene>. <b><span class='text-gray'>The C-terminal domain</span></b> carries the <scene name='10/1050300/Phac_i_catalytic_site/2'>catalytic site, formed by the aminoacid triad</scene> <b><span class='text-red'>(Cys-Asp-His)</span></b> located deep within the <b><span class='text-gray'>hydrophobic</span></b> <scene name='10/1050300/Phac_i_polar/1'>cavity</scene>, that in the closed conformation is partially covered by the <b><span class='text-orange'>Cap</span></b> <scene name='10/1050300/Phac_i_cap_subdomain/1'>subdomain</scene>.<ref name='Neoh' /><ref name='Chek' /> | ||
| + | |||
| + | Structure from two class 1 phaCs ([https://proteopedia.org/wiki/index.php/5hz2 PhaC<sub>cn</sub>-CAT] and [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT]). Image obtained from Chek et., al 2018.<ref name='Chek' /> | ||
| + | [[Image:Phac.png]] | ||
=== N-terminal domain === | === N-terminal domain === | ||
| Line 25: | Line 28: | ||
| - | Video produced by Chek et al., | + | Video produced by Chek et al., 2020, showing the phaC conformational change.<ref name='Chek_20'>CHEK, Min Fey; KIM, Sun-Yong; MORI, Tomoyuki; TAN, Hua Tiang; SUDESH, Kumar; HAKOSHIMA, Toshio. Asymmetric Open-Closed Dimer Mechanism of Polyhydroxyalkanoate Synthase PhaC. Iscience, [S.L.], v. 23, n. 5, p. 101084, maio 2020. Elsevier BV. http://dx.doi.org/10.1016/j.isci.2020.101084.</ref> |
<html5media height='337' width='600'>https://www.youtube.com/watch?v=LALMBSB4Bts</html5media> | <html5media height='337' width='600'>https://www.youtube.com/watch?v=LALMBSB4Bts</html5media> | ||
| Line 36: | Line 39: | ||
[[Image:Proteins-cs.jpg]] | [[Image:Proteins-cs.jpg]] | ||
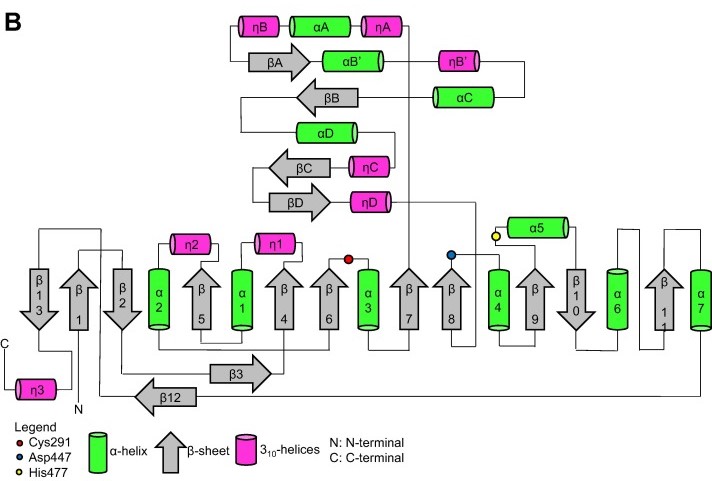
| - | === | + | === Terciary structure === |
| - | + | Terciary structure of [https://proteopedia.org/wiki/index.php/5hz2 PhaC<sub>cn</sub>-CAT] and [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT], with the Cap subdomain highlighted. Image obtained from Neoh et al., 2022.<ref name='Neoh' /> | |
[[Image:Proteins-quaternary.jpg]] | [[Image:Proteins-quaternary.jpg]] | ||
| + | |||
| + | |||
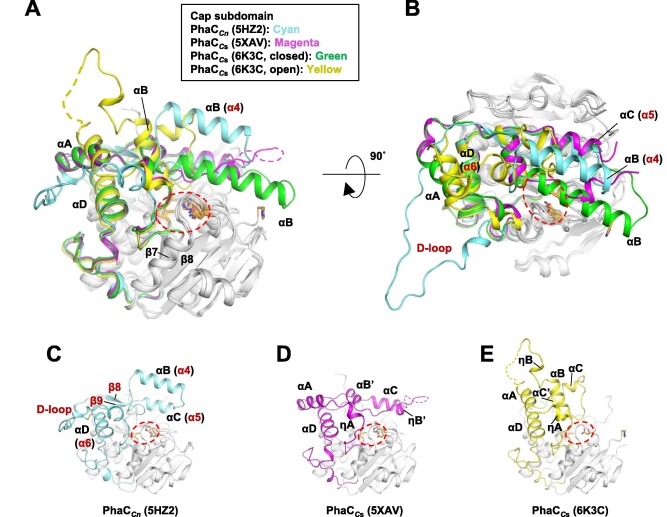
| + | === Quaternary structure === | ||
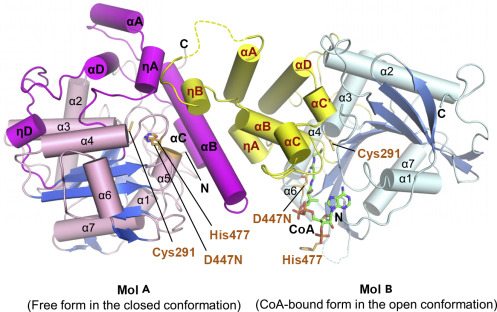
| + | The co-crystal of a [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT] with CoA reveals the complex structure of an asymmetric dimer, with two protomers in different forms. The closed form is CoA-free, and the open form is CoA-bound. In the open form, the CoA molecule is accommodated in the active site cleft, created by dynamic conformational transitions in the <b><span class='text-orange'>Cap</span></b> <scene name='10/1050300/Phac_i_catalytic_cap_sub/1'>subdomain</scene> and local conformational shifts in the <b><span class='text-cyan'>α/β-hydrolase core</span></b> <scene name='10/1050300/Phac_i_catalytic_hydrolase_sub/1'>subdomain</scene>. One protomer was in the closed conformation (free form), while the other protomer was bound to CoA in an open conformation. Observations were made that the free form is important for stabilizing the form bound to CoA.<ref name='Neoh' /><ref name='Chek_20' /> | ||
| + | |||
| + | In general, monomeric and dimeric forms exist in equilibrium, but it is generally understood that PhaC is active in its dimeric form, which is essential for the functionality of PHA synthase. This active form (dimeric form) can be induced in the presence of substrates.<ref name='Neoh' /> | ||
| + | |||
| + | The <b><span class='text-orange'>Cap</span></b> <scene name='10/1050300/Phac_i_catalytic_cap_sub/1'>subdomain</scene> and mainly its LID region are important in the dimerization of PhaC, as they mediate the movement of the protomer, regulating the shift between the closed-closed form (homodimer) and the open-closed form (heterodimer).<ref name='Neoh' /> | ||
| + | |||
| + | Structure of the [https://proteopedia.org/wiki/index.php/6k3c PhaC<sub>cs</sub>-CAT] heterodimer formed by the free and CoA-bound forms. Image obtained from Chek et., al 2020.<ref name='Chek_20' /> | ||
| + | [[Image:Open close phaC.jpg]] | ||
== Class 2 == | == Class 2 == | ||
Revision as of 00:58, 3 June 2024
Introduction
| |||||||||||