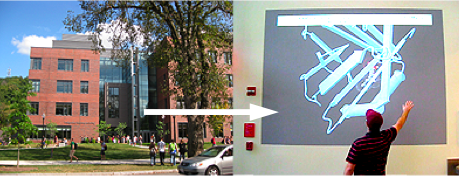
Molecular Playground/ Copper-Zinc Superoxide Dismutase
From Proteopedia
| Molecular Playground at the University of Massachusetts. MOVIE. |
Proposed Article Title: Molecular Playground/Copper-Zinc Superoxide Dismutase
Banner: Cu/ Zn Superoxide Dismutase keeps you young
The important function of Cu/ Zn superoxide dismutase (SOD) is to detoxify damaging forms of oxygen. It catalyzes the dismutation of superoxide (O2-) anion into molecular oxygen (O2) and hydrogen peroxide (H2O2)[1]. Mutations or disruptions in the protein can exacerbate a number of diseases, such as amyotrophic lateral sclerosis (ALS) and diabetes[2]. One of the reported mutations involves the reduction of the disulfide bond, leading to a destabilized protein structure [2]. This mutation is featured in the fatal ALS disease. The motor neurons of individuals are affected, and voluntary muscle control is lost.
|
Human Cu/ Zn superoxide dismutases (SOD) are homodimer proteins, consisting of two identical monomers, found in the cytoplasm of cells. Each monomer contains . Each Cu is bound to and each Zn is bound to four atoms as well. A is also located in each monomer . Another interesting feature that contributes to the stability of the protein is the tight between the monomers and “the two halves of the βeta (β)- barrel core” ( insert reference). Hydrophobic, Polar
References
- ↑ Superoxide dismutase in Wikipedia
- ↑ 2.0 2.1 Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006 Jul;1763(7):747-58. Epub 2006 May 17. PMID:16828895 doi:10.1016/j.bbamcr.2006.05.003