Image:Imatinib bound to receptor.PNG
From Proteopedia
No higher resolution available.
Imatinib_bound_to_receptor.PNG (529 × 221 pixel, file size: 9 KB, MIME type: image/png)
Licensing
{{subst:No license from license selector|Don't know}}
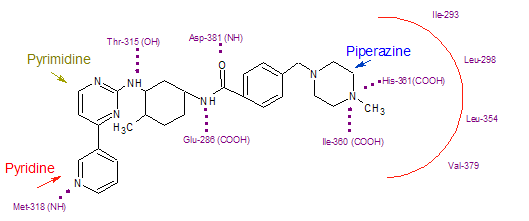
Imatinib binds to Abl domain via six hydrogen bond interactions. This stabilizes the imatinib Bcr-Abl complex and prevents ATP from reaching its binding site. The hydrogen bonds involve the pyridine-N and backbone-NH of Met-318, the aminopyrimidine and side chain hydroxyl of Thr-315, the amide-NH and side chain carboxylate of Glu-285, the carbonyl and backbone-NH of Asp-381, the protonated methylpiperazine with the backbone-carbonyl atoms of Ile-360 and His-361. Additionally, a number of van der Waals interactions contribute to binding. A hydrophobic pocket is formed by amino acid residues Ile-293, Leu-298, Leu-354 and Val-379 around the phenyl ring adjacent to the piperazinyl-methyl group of imatinib. At the time of its discovery, in the absence of structural information, no clear explanation for the impressive selectivity of imatinib could be found
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 18:24, 9 December 2012 | Cristina Murga (Talk | contribs) | 529×221 | 9 KB |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
