This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
SARS-CoV-2 Papain-Like protease (PLpro)
From Proteopedia
|
Contents |
SARS-CoV-2 Papain-Like protease (PLpro)
Introduction
Papain-like protease () from SARS-CoV-2 is a 315 amino acids long cysteine protease is found in the large multi-domain membrane-bound non-structural protein 3 (nsp3)(1945 residues) between the SARS domain (SUD/HVR) and a nucleic acid-binding domain (NAD) (Frick et al, 2020, OSIPIUK et al., 2021). It has a high percentage of (highlighted in pink)(3.5%) (OSIPIUK et al., 2021, YOSHIMOTO, 2020).
SARS-CoV-2 polyprotein
The first SARS-CoV-2 open reading frame (ORF) codes for one large polyprotein, 1ab (7096 aminoacids) or 1a (4405 amino acids) built by fifteen non-coding proteins including the two proteases responsible to cleave the polyprotein: Main protease (Mpro, also called 3C-like protease SARS-CoV-2_Coronavirus_Main_Protease[[1]] and Papain-Like protease (OSIPIUK et al., 2021, YOSHIMOTO, 2020).
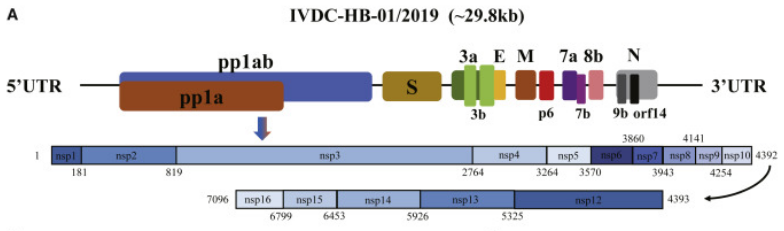
 Wu et al, 2020. https://doi.org/10.1016/j.chom.2020.02.001
Wu et al, 2020. https://doi.org/10.1016/j.chom.2020.02.001
Ullrich, Nitsche, 2020. https://doi.org/10.1016/j.bmcl.2020.127377
Structure
|
PLpro active site is represented by C111S and contains the canonical protease catalytic triad composed by . Differing from the dyad of Mpro, composed of Cys145 and His41. For PLpro Cys111 acts as a nucleophile and Asp286 promotes deprotonation of His272 (OSIPIUK et al., 2021). The residue C11 is 3.6 Å from H272 and the hydrogen bond donated by H272 to D286 has a 3.0 Å distance. (GAO et al., 2021)
The structure of SARS-CoV-2 PLpro is a . It has an N-terminal ubiquitin-like (Ubl, β1-3) domain composed of five β-stands, one α-helices, and one 310-helix formed by 1 to 60 residues. The catalytic domain is comprised of . The first subdomain is the composed of six α-helices (α2-7) and a small β-hairpin. The second subdomain is the (β8-13), composed of six β-strands and the catalytic residues Cys111, His272, and Asp286 located between the thumb and palm subdomains. The third and last subdomain is the (or β-stranded finger), the most complex one, it has six β-strands and two α-helices and includes a ("zinc ribbon" fold group) coordinated by cysteines 189, 192, 224, and 266 located on two loops of 2 β-hairpins essential for protease activity (β4-7) (OSIPIUK et al., 2021, GAO et al., 2021)
The structure and conformational change of the located between β11-12 plays an interesting role in the substrate binding, it recognizes P2-P4 and when the ligand is not present it presents a relatively close conformation, but when it is present the loop moves to provide space to the tetrapeptide, this can be seen with the tetrapeptidade , and constitutes an interesting property for a drug target. This plasticity suggests an induced-fit mechanism, making it possible for PLpro to recognize different substrates. But the BL2 loop is sensitive for the size, big substrates promote a more close configuration physically blocking the active site, a characteristic that can be seen with (FU et al., 2021) and that restricts the possibilities of drugs that can be used against COVID-19 that objective contains PLpro activity (GAO et al., 2021, YADAV et al., 2021).
Functions
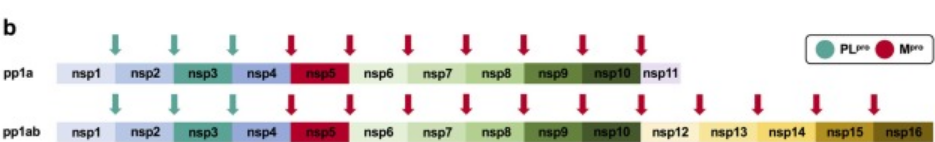
The main or at least better-known function of SARS-CoV-2 PLpro is the cleavage and maturation of viral large polyproteins 1a and 1ab. PLpro cleaves at three sites: Between Nsp1 and Nsp2, Nsp2 and Nsp3, and between nsp3 and nsp4, liberating non-structural proteins 1, 2 ad 3 using the catalytic domain, palm subdomain, and triad catalytic (LI; ZHANG, 2021).
But it has other important functions that play a central role especially on viral replication and proteins maturation (OSIPIUK et al., 2021). Some of its functions are correlated to immune evasion specifically the ones connected to deubiquitinations (reversing the ubiquitination by reversing post-transnational modifications) (BOSKEN et al., 2020) and ISGylation (interferon-stimulates gene product 15) events (SHIN, 2020), maturation, and assembly of RTC (ER membrane-bound multi-component replicase, transcriptase complex) by the Ubiquitin domain, inactivating pathways correlated to innate immune system response and probably acts on host cells facilitating viral replication (OSIPIUK et al., 2021, GAO et al., 2021). These modifications upregulate the production of cytokines, chemokines, and other products correlated with host viral inhibition and facilitate the inflammation process (YADAV et al., 2021, 2020, SHIN, 2020).
All these functions put PLpro as a potential aim to drug development, once they have a relevant impact on virus pathogenesis (SHIN, 2020).
Recognition site
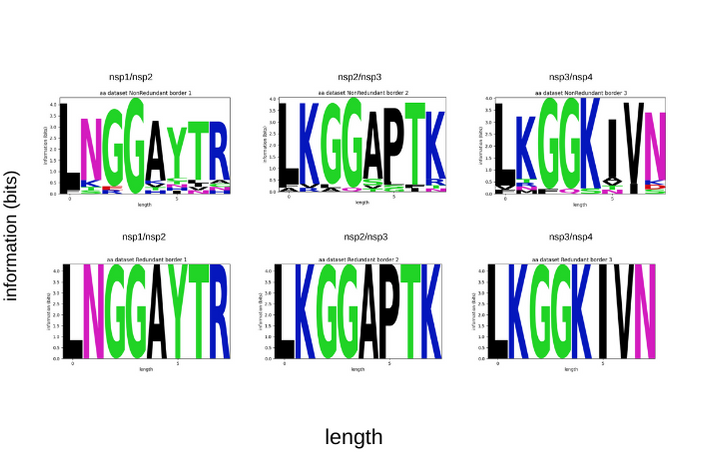
The site where the sequence, ligand, or inhibitor binds is identified by S1-S4 and the amino acids that bind there are represented by P4-P1 The consensus sequence for PLpro is (LXGG XXXX), more specifically, for the bordering between Nsp1 and Nsp 2 that sequence is (LNGG AYTR), between Nsp2 and Nsp3 (LKGG APTK), and for Nsp3 and Nsp4 (LKGG KIVN) (GAO et al., 2021, OSIPIUK et al., 2021). The motif that binds to the P4-P1 substrate is LXGG (OSIPIUK et al., 2021).
These sequences tend to be well-conserved, and that the change can lead to non-recognition by the protease affecting the viral replication. This and the central role of PLpro functions in viral reproduction lead this protein to be one of the main drug targets for development for COVID-19 (LI; ZHANG, 2021). Despite this analyzing 209,662 complete genome sequences submitted on the GISAID database [2] in the period between the December 7th, 2019 and December 16th, 2020, variations on these conserved sequences were found and are presented in Sequence Logos graphs by cleavage site constructed using non redundant data (to evaluate the variations found) and redundant data(to evaluate the conservation), respectively. More information avaiable in https://github.com/labbces/SARS-CoV-2-ProtPhylos[[3]] (Computational, Evolutionary and Sistems Biology Laboratory - CENA/USP)
When the inhibitor is bound to the active site, a mobile β-turn/loop comprised of Gly266 to Gly271 part of the Catalytic domain and Palm subdomain located near adjacent to the active site closes upon the substrate (OSIPIUK et al., 2021).
Similarity
|
As SARS-CoV and MERS-CoV, SARS-CoV-2 codes a single papain-like protease (PLpro). Other CoV viruses (Mouse hepatitis virus - MHV, NL63, OC43, HKU1, and 229E) produce two of these protease.
Different from NSP3 as a whole, PLpro are conserved is SARS-CoV-2, SARS-CoV, MERS-CoV, and Swine Acute Diarrhea Syndrome (SADS), it has a similarity of 90% with (and 82-83% identical percentage), 49% with (and 31% identical percentage) and are more distinct to PLpro (Where Violet represents SADS PLpro and light blue represents SARS-CoV-2 PLpro)(OSIPIUK et al., 2021).
In terms of structure, PLpro of these different viruses tends to be similar with punctual dissimilarities that can cause some function or substrate preferences. Comparing the functions, SARS-CoV PLpro prefers the ubiquitinated substrates while SARS-CoV-2 PLpro can act to ubiquitinated and ISGylated substrates/proteins, showing a preference for the second one. This dual functionality also highlights this protease as a possible important drug target (GAO et al., 2021).
Subdomains
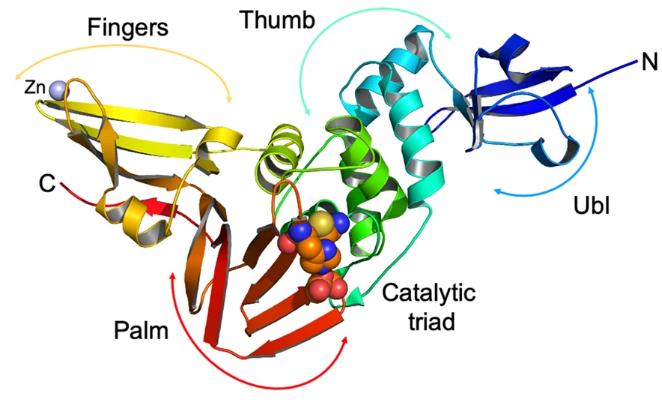 (OSIPIUK, Jerzy et al, 2021. https://doi.org/10.1038/s41467-021-21060-3)
(OSIPIUK, Jerzy et al, 2021. https://doi.org/10.1038/s41467-021-21060-3)
References
1. GAO, Xiaopan *et al*. Crystal structure of SARS-CoV-2 papain-like protease. **Elsevier**: Acta Pharmaceutica Sinica B, [*s. l.*], v. 11, p. 237-245, 2021. DOI [4]. Disponível em: [5]. Acesso em: 1 dez. 2021.
2. OSIPIUK, Jerzy et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nature: Communications, [s. l.], v. 12, n. 743, 2021. DOI [6]. Disponível em: [7]. Acesso em: 11 nov. 2021.
3. GAO, Xiaopan et al. Crystal structure of SARS-CoV-2 papain-like protease. Elsevier: Acta Pharmaceutica Sinica B, [s. l.], v. 11, p. 237-245, 2021. DOI [8]. Disponível em: [9]. Acesso em: 1 dez. 2021.
4. FREITAS, Brendan T. et al. Characterization and Noncovalent Inhibition of the Deubiquitinase and deISGylase Activity of SARS-CoV-2 Papain-Like Protease. ACS Infectious Diseases, [s. l.], 2020. DOI [10]. Disponível em: [11]. Acesso em: 8 dez. 2021.
5. YADAV, Rohitash et al. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells: MDPI, [s. l.], v. 10, n. 4, ed. 821, 2021. DOI https://doi.org/10.3390/cells10040821. Disponível em: https://www.mdpi.com/2073-4409/10/4/821. Acesso em: 3 nov. 2021.
6. YOSHIMOTO, Francis K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus‑2 (SARS CoV‑2 or n‑COV19), the Cause of COVID‑19. The protein Journal: Springer Link, [s. l.], 2020. DOI https://doi.org/10.1007/s10930-020-09901-4. Disponível em: https://link.springer.com/article/10.1007%2Fs10930-020-09901-4. Acesso em: 19 nov. 2021.
7. BOSKEN, Yuliana K. et al. Insights Into Dynamics of Inhibitor and Ubiquitin-Like Protein Binding in SARS-CoV-2 Papain-Like Protease. Frontiers: in Molecular Biosciences, [s. l.], 2020. DOI https://doi.org/10.3389/fmolb.2020.00174. Disponível em: https://www.frontiersin.org/articles/10.3389/fmolb.2020.00174/full. Acesso em: 10 dez. 2021.
8. LI, Daoqun; ZHANG, Leiliang. Molecular docking of potential SARS-CoV-2 papain-like protease inhibitors. Biochemical and Biophysical Research Communications: Elsevier, [s. l.], v. 538, p. 72-79, 2021. DOI https://doi.org/10.1016/j.bbrc.2020.11.083. Disponível em: https://www.sciencedirect.com/science/article/pii/S0006291X20321264?via%3Dihub. Acesso em: 2 dez. 2021.
9. SHIN, Donghyuk. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature, [s. l.], 2020. DOI https://doi.org/10.1038/s41586-020-2601-5. Disponível em: https://www.nature.com/articles/s41586-020-2601-5. Acesso em: 25 nov. 2021.
10. FU, Ziyang et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nature: Communications, [s. l.], 2021. DOI https://doi.org/10.1038/s41467-020-20718-8. Disponível em: https://www.nature.com/articles/s41467-020-20718-8. Acesso em: 6 nov. 2021.
11. WU, Aiping et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host & Microbe, [s. l.], 2020. DOI https://doi.org/10.1016/j.chom.2020.02.001. Disponível em: https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(20)30072-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS193131282030072X%3Fshowall%3Dtrue. Acesso em: 1 out. 2021.