Sandbox 156
From Proteopedia
(→Chloramphenicol Acetyltransferase Type III) |
(→Chloramphenicol Acetyltransferase Type III) |
||
| Line 1: | Line 1: | ||
= Chloramphenicol Acetyltransferase Type III = | = Chloramphenicol Acetyltransferase Type III = | ||
| - | Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of an acetyl group from acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein of about 25 000 k-Da. | + | Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of an acetyl group from [[acetyl-CoA]] to hydroxyl groups of [[chloramphenicol]]. CAT III is a trimeric protein of about 25 000 k-Da. |
== Introduction == | == Introduction == | ||
| Line 18: | Line 18: | ||
==Structure== | ==Structure== | ||
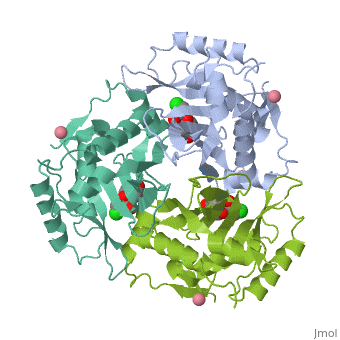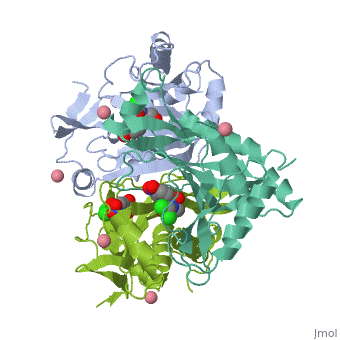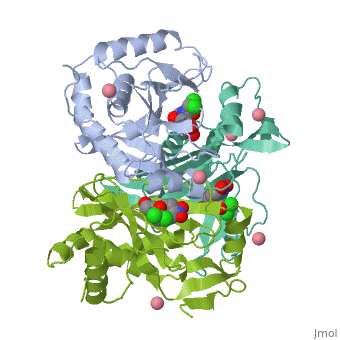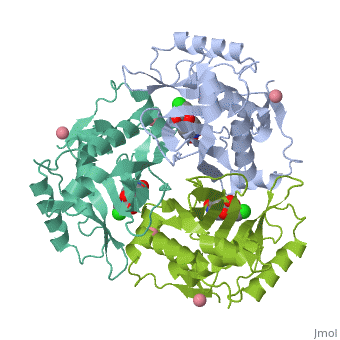
<applet load='4CLA' size='300' frame='true' align='right' caption='' /> | <applet load='4CLA' size='300' frame='true' align='right' caption='' /> | ||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 05:09, 21 March 2010
Contents |
Chloramphenicol Acetyltransferase Type III
Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of an acetyl group from acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein of about 25 000 k-Da.
Introduction
The bacterial enzyme CAT III
Reaction of CAT III
In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a nucleophilic attack from the deprotonated oxygen to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another CAT III catalyzed nucleophilic attack to another acetyl-CoA and a 1,3-diacetylchloramphenicol product is formed. [1]
Structure
|
References
- ↑ Murray IA, Lewendon A, Williams JA, Cullis PM, Shaw WV, Leslie AG. Alternative binding modes for chloramphenicol and 1-substituted chloramphenicol analogues revealed by site-directed mutagenesis and X-ray crystallography of chloramphenicol acetyltransferase. Biochemistry. 1991 Apr 16;30(15):3763-70. PMID:2015231
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |