Sandbox 156
From Proteopedia
(→Introduction) |
|||
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
| - | Found in bacteria, the CAT III enzyme is responsible for conferring resistance to chloramphenicol. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and inhibiting [[peptidyl transferase]] activity <ref>PMID: 1544895</ref>. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The enzyme consists of three identical subunits with three active sites at their interface. The side chains of one subunit allow van der Waals contacts and two hydrogen bonds with chloramphenicol and acetyl-CoA, causing binding of the substrates. The opposing subunit provides a histidine (His-195) residue essential for catalysis<ref>PMID: 8407936</ref>. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue<ref>PMID:2015231</ref>. | + | <applet load='4CLA' size='300' frame='true' align='right' caption='' /> |
| + | |||
| + | Found in bacteria, the CAT III enzyme is responsible for conferring resistance to chloramphenicol. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and inhibiting [[peptidyl transferase]] activity <ref>PMID: 1544895</ref>. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a numerous bacterial species<ref>PMID: 2268277</ref>. | ||
| + | |||
| + | The enzyme consists of three identical subunits with three active sites at their interface. The side chains of one subunit allow van der Waals contacts and two hydrogen bonds with chloramphenicol and acetyl-CoA, causing binding of the substrates. The opposing subunit provides a histidine (His-195) residue essential for catalysis<ref>PMID: 8407936</ref>. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue<ref>PMID:2015231</ref>. | ||
=== Reaction of CAT III === | === Reaction of CAT III === | ||
Revision as of 05:56, 21 March 2010
Contents |
Chloramphenicol Acetyltransferase Type III
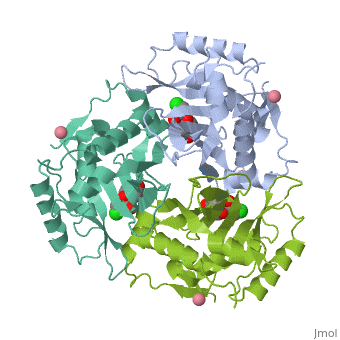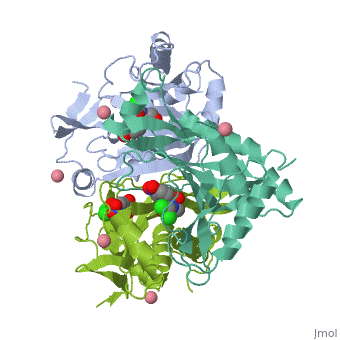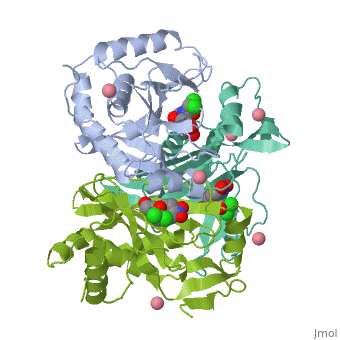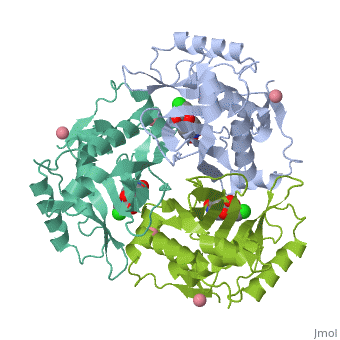
Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of an acetyl group from acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein of about 25 000 k-Da.
Introduction
|
Found in bacteria, the CAT III enzyme is responsible for conferring resistance to chloramphenicol. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and inhibiting peptidyl transferase activity [1]. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a numerous bacterial species[2].
The enzyme consists of three identical subunits with three active sites at their interface. The side chains of one subunit allow van der Waals contacts and two hydrogen bonds with chloramphenicol and acetyl-CoA, causing binding of the substrates. The opposing subunit provides a histidine (His-195) residue essential for catalysis[3]. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue[4].
Reaction of CAT III
In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a nucleophilic attack from the oxyanion to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another CAT III catalyzed nucleophilic attack to another acetyl-CoA and a 1,3-diacetylchloramphenicol product is formed. [5]
Structure
|
References
- ↑ Day PJ, Shaw WV. Acetyl coenzyme A binding by chloramphenicol acetyltransferase. Hydrophobic determinants of recognition and catalysis. J Biol Chem. 1992 Mar 15;267(8):5122-7. PMID:1544895
- ↑ Lewendon A, Shaw WV. Elimination of a reactive thiol group from the active site of chloramphenicol acetyltransferase. Biochem J. 1990 Dec 1;272(2):499-504. PMID:2268277
- ↑ Lewendon A, Shaw WV. Transition state stabilization by chloramphenicol acetyltransferase. Role of a water molecule bound to threonine 174. J Biol Chem. 1993 Oct 5;268(28):20997-1001. PMID:8407936
- ↑ Murray IA, Lewendon A, Williams JA, Cullis PM, Shaw WV, Leslie AG. Alternative binding modes for chloramphenicol and 1-substituted chloramphenicol analogues revealed by site-directed mutagenesis and X-ray crystallography of chloramphenicol acetyltransferase. Biochemistry. 1991 Apr 16;30(15):3763-70. PMID:2015231
- ↑ Murray IA, Lewendon A, Williams JA, Cullis PM, Shaw WV, Leslie AG. Alternative binding modes for chloramphenicol and 1-substituted chloramphenicol analogues revealed by site-directed mutagenesis and X-ray crystallography of chloramphenicol acetyltransferase. Biochemistry. 1991 Apr 16;30(15):3763-70. PMID:2015231
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |