Adenylosuccinate Synthetase
From Proteopedia
(→Introduction) |
|||
| Line 6: | Line 6: | ||
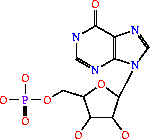
Adenylosuccinate Synthetase (AdSS) is part of the ligase family of enzymes. Its systematic name is IMP:L-aspartate ligase. It is mainly involved in purine bio-synthesis. It does this by catalyzing the GTP dependent changeover of IMP and aspartic acid to AMP in the presense of Mg<sup>2+</sup>.<ref>PMID:1698173 </ref> In Humans, AdSS catalyzes the first committed step in the purine nucleotide cycle by the de novo synthesis of adenosine monophosphate.<ref>PMID:649264</ref> | Adenylosuccinate Synthetase (AdSS) is part of the ligase family of enzymes. Its systematic name is IMP:L-aspartate ligase. It is mainly involved in purine bio-synthesis. It does this by catalyzing the GTP dependent changeover of IMP and aspartic acid to AMP in the presense of Mg<sup>2+</sup>.<ref>PMID:1698173 </ref> In Humans, AdSS catalyzes the first committed step in the purine nucleotide cycle by the de novo synthesis of adenosine monophosphate.<ref>PMID:649264</ref> | ||
| - | AdSS (3hid) was isolated from <i>Yersinia pestis</i> CO92 and can be found in a variety of organisms ranging from yeast to bacteria to humans. The gene is located on chromosome 1 q44, in humans. | + | AdSS (3hid) was isolated from <i>Yersinia pestis</i> CO92 and can be found in a variety of organisms ranging from yeast to bacteria to humans. The gene is located on chromosome 1 q44, in humans and is expressed in the majority of an organisms cells. |
==Structure== | ==Structure== | ||
Revision as of 01:20, 29 March 2010
Adenylosuccinate Synthetase
Contents |
Introduction
Adenylosuccinate Synthetase (AdSS) is part of the ligase family of enzymes. Its systematic name is IMP:L-aspartate ligase. It is mainly involved in purine bio-synthesis. It does this by catalyzing the GTP dependent changeover of IMP and aspartic acid to AMP in the presense of Mg2+.[1] In Humans, AdSS catalyzes the first committed step in the purine nucleotide cycle by the de novo synthesis of adenosine monophosphate.[2]
AdSS (3hid) was isolated from Yersinia pestis CO92 and can be found in a variety of organisms ranging from yeast to bacteria to humans. The gene is located on chromosome 1 q44, in humans and is expressed in the majority of an organisms cells.
Structure
The AdSS enzyme is a dimer consisting of two identical monomeric subunits. The main structural component of each monomer is a centrally located beta sheet that is comprised of 10 strands. Nine of the 10 strands are parallel while the 10th strand is anti-parallel with respect to the other 9 strands. There are also several other secondary structures including 2 small 3/10 helices, two anti-parallel sheets consisting of 2 and 3 strands respectively. Additionally there are 11 alpha helices.[3] AdSS has three major binding sites, one for ACT, one for GTD and one for IMP.
Helices are highlighted in green and beta sheets are shown in orange .
AdSS has an optimal pH of 6.5 and a denatures at pH 7.4.[4] It has a melting point of 85 degrees Celsius.[5]
Reaction Mechanism
AdSS undergoes the following amination reaction:
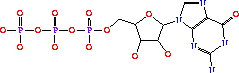
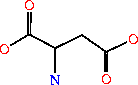
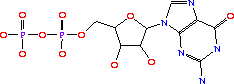
GTP + IMP + L-Asp -> GDP + phosphate + N6-(1,2-dicarboxyethyl)-AMP[6]
The 6-O of inosine is displaced by aspartate which yields adenylosuccinate. The presence, or over abundance of AMP acts as a feedback inhibitor for AdSS.[7]
Resulting Diseases
Defects in the gene product can result in a wide variety of diseases.
References
- ↑ Mukhopadhyay RP, Chandra AL. Keratinase of a streptomycete. Indian J Exp Biol. 1990 Jun;28(6):575-7. PMID:1698173
- ↑ Pierloot RA. The treatment of psychosomatic disorders by the general practitioner. Int J Psychiatry Med. 1977-1978;8(1):43-51. PMID:649264
- ↑ Poland BW, Silva MM, Serra MA, Cho Y, Kim KH, Harris EM, Honzatko RB. Crystal structure of adenylosuccinate synthetase from Escherichia coli. Evidence for convergent evolution of GTP-binding domains. J Biol Chem. 1993 Dec 5;268(34):25334-42. PMID:8244965
- ↑ Ware JD, Bellini WJ, Ash RJ. Metabolic characteristics of cells infected with a herpesvirus of turkeys. J Natl Cancer Inst. 1975 Dec;55(6):1379-82. PMID:1548
- ↑ Katsarkas A, Kirkham TH. Paroxysmal positional vertigo--a study of 255 cases. J Otolaryngol. 1978 Aug;7(4):320-30. PMID:691098
- ↑ Van der Weyden MB, Kelly WN. Human adenylosuccinate synthetase. Partial purification, kinetic and regulatory properties of the enzyme from placenta. J Biol Chem. 1974 Nov 25;249(22):7282-9. PMID:4436310
- ↑ Bates PC, Millward DJ. Muscle growth and protein turnover in a fast growing rat strain. Proc Nutr Soc. 1978 May;37(1):19A. PMID:662843
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Proteopedia Page Contributors and Editors (what is this?)
Aaron Smith, Michal Harel, Alexander Berchansky, David Canner, Andrea Gorrell