AChE inhibitors and substrates
From Proteopedia
| Line 33: | Line 33: | ||
<StructureSection load='Soman1.pdb' size='500' frame='true' align='right' scene='2wfz/Al/1' > | <StructureSection load='Soman1.pdb' size='500' frame='true' align='right' scene='2wfz/Al/1' > | ||
| + | |||
| + | ==== Soman ==== | ||
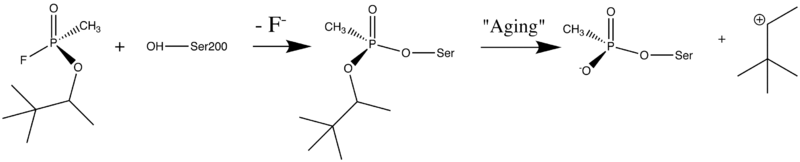
As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation ("aging")</scene> of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation ("aging")</scene> of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | ||
At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <font color='yellow'><b>yellow</b></font>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <font color='pink'><b>pink</b></font>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <font color='yellow'><b>yellow</b></font>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <font color='pink'><b>pink</b></font>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | ||
| Line 41: | Line 43: | ||
{{Clear}} | {{Clear}} | ||
| + | ====Sarin==== | ||
<scene name='1cfj/Cv/1'>Sarin.</scene> [http://en.wikipedia.org/wiki/Sarin Sarin], O-''iso''propylmethylphosponofluoridate, is an other toxic OP compound. It is also inhibits AChE by covalent binding to the catalytic Ser200. The active sites of aged <scene name='1cfj/Cv/2'>sarin-TcAChE</scene> ([[1cfj]]) and aged soman-TcAChE ([[1som]] and [[2wg0]]) are almost identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the ACh. | <scene name='1cfj/Cv/1'>Sarin.</scene> [http://en.wikipedia.org/wiki/Sarin Sarin], O-''iso''propylmethylphosponofluoridate, is an other toxic OP compound. It is also inhibits AChE by covalent binding to the catalytic Ser200. The active sites of aged <scene name='1cfj/Cv/2'>sarin-TcAChE</scene> ([[1cfj]]) and aged soman-TcAChE ([[1som]] and [[2wg0]]) are almost identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the ACh. | ||
There are four hydrogen bond donors <font color='red'><b>(red dotted lines)</b></font> to the anionic phosphonyl oxygen atoms: the backbone amide nitrogen atoms of Ala201, Gly118, and Gly119, as well as His440 Nε2. The sarin <font color='cyan'><b>methyl carbon (colored cyan)</b></font> is within non-bonded contact distances '''(black dotted lines)''' of Phe288 and Phe290 in the acyl binding pocket <ref name="Millard"/>. | There are four hydrogen bond donors <font color='red'><b>(red dotted lines)</b></font> to the anionic phosphonyl oxygen atoms: the backbone amide nitrogen atoms of Ala201, Gly118, and Gly119, as well as His440 Nε2. The sarin <font color='cyan'><b>methyl carbon (colored cyan)</b></font> is within non-bonded contact distances '''(black dotted lines)''' of Phe288 and Phe290 in the acyl binding pocket <ref name="Millard"/>. | ||
{{Clear}} | {{Clear}} | ||
| + | ====DFP==== | ||
<scene name='2dfp/Cv/3'>DFP</scene>, di''iso''propylphosphorofluoridate, is an other toxic OP nerve agent. It is also inhibits AChE by covalent binding to the catalytic Ser200. As in the case with soman ([[1som]]) and sarin ([[1cfj]]), there are four hydrogen bond donors (dotted lines) to the anionic phosphonyl oxygen atoms: the backbone amide nitrogen atoms of Ala201, Gly118, and Gly119, as well as His440 Nε2 at the <scene name='2dfp/Cv/4'>active site</scene> of aged DFP-TcAChE ([[2dfp]]). Phosphorylation with DFP caused an unexpected distortion in the main chain of a loop that includes residues F288 and F290 of the TcAChE acyl binding pocket. F288 and F290 move significantly in the <font color='lime'><b>DFP-TcAChE structure (lime)</b></font>, in comparison to their positions in the <font color='magenta'><b>native enzyme</b></font> ([[2ace]]). This is the first major conformational change reported in the active site of any AChE−ligand complex, and it offers a structural explanation for the substrate selectivity of AChE <ref name="Millard"/>. | <scene name='2dfp/Cv/3'>DFP</scene>, di''iso''propylphosphorofluoridate, is an other toxic OP nerve agent. It is also inhibits AChE by covalent binding to the catalytic Ser200. As in the case with soman ([[1som]]) and sarin ([[1cfj]]), there are four hydrogen bond donors (dotted lines) to the anionic phosphonyl oxygen atoms: the backbone amide nitrogen atoms of Ala201, Gly118, and Gly119, as well as His440 Nε2 at the <scene name='2dfp/Cv/4'>active site</scene> of aged DFP-TcAChE ([[2dfp]]). Phosphorylation with DFP caused an unexpected distortion in the main chain of a loop that includes residues F288 and F290 of the TcAChE acyl binding pocket. F288 and F290 move significantly in the <font color='lime'><b>DFP-TcAChE structure (lime)</b></font>, in comparison to their positions in the <font color='magenta'><b>native enzyme</b></font> ([[2ace]]). This is the first major conformational change reported in the active site of any AChE−ligand complex, and it offers a structural explanation for the substrate selectivity of AChE <ref name="Millard"/>. | ||
Revision as of 10:34, 28 November 2010

Dear readers, this page presents only a small part of the great world of the acetylcholinesterase inhibitors. So, please see also our pages AChE inhibitors and substrates (Part II), AChE inhibitors and substrates (Part III), AChE bivalent inhibitors and AChE bivalent inhibitors (Part II). The images at the left and at the right correspond to one representative acetylcholinesterase with substrate structure, i.e. crystal structure of Torpedo californica acetylcholinesterase with acetylcholine (2ace).
Contents |
AChE substrate
|
Solution of the three-dimensional (3D) structure of Torpedo californica acetylcholinesterase (TcAChE) in 1991 [1] opened up new horizons in research on an enzyme that had already been the subject of intensive investigation. The unanticipated structure of this extremely rapid enzyme, in which the active site was found to be buried at the bottom of a , lined by (colored dark magenta), led to a revision of the views then held concerning substrate traffic, recognition and hydrolysis [2]. This led to a series of theoretical and experimental studies, which took advantage of recent advances in theoretical techniques for treatment of proteins, such as molecular dynamics and electrostatics and to site-directed mutagenesis, utilizing suitable expression systems. Acetylcholinesterase hydrolysizes the neurotransmitter acetylcholine , producing group. directly binds (via its nucleophilic Oγ atom) within the catalytic triad (ACh/TcAChE structure 2ace). The residues are also important in the ligand recognition [3].
AChE monovalent inhibitors
Organophosphorus acid anhydride nerve agents
Organophosphorus (OP) acid anhydride nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated.
| |||||||||||
Treatment of Alzheimer's disease
Please see pages AChE inhibitors and substrates (Part II) and AChE inhibitors and substrates (Part III).
AChE bivalent inhibitors
Please see pages AChE bivalent inhibitors and AChE bivalent inhibitors (Part II)
Selected 3D Structures of AChE
- 2ace This is the original solved structure for Torpedo Californica
- 1ea5 This is one of the highest quality representative X-ray structures in the PDB.
- 1eve The E2020 (Aricept) complex.
- 1ax9 Endrophonium complex.
- 1vot Complex with Huperzine, a Chinese folk medicine.
- 1fss Complex with snake venum toxin Fasciculin-II.
- 1acj Complex with tacrine.
- 1e66 Complex with huprine X.
- 1dx6 Complex with galanthamine.
- 1qti Complex with galanthamine.
- 1w6r Complex with galanthamine iminium derivative.
- 2ack Complex with edrophonium.
- 1vzj Structure of the tetramerization domain of acetylcholinesterase.
- 1gqr Complex with rivastigmine.
- 1gqs Complex with NAP alone.
- 1vxr Complex with VX.
- 2vja Complex with OTMA.
- 1som Complex with soman.
- 2wfz Complex with nonaged soman.
- 2wg0 Complex with aged soman.
- 2wg1 Complex with aged soman and 2-PAM.
- 1cfj Complex with aged sarin.
- 2dfp Complex with aged DFP.
Additional Resources
For additional information, see: Alzheimer's Disease
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
- ↑ 5.0 5.1 5.2 Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:10353814 doi:http://dx.doi.org/10.1021/bi982678l
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Joel L. Sussman, Michal Harel, Jaime Prilusky, David Canner