Beta secretase
From Proteopedia
| Line 1: | Line 1: | ||
| - | <applet load="1w51" size=" | + | <applet load="1w51" size="350" color="white" frame="true" align="right" caption="β-Secretase" scene="Beta_secretase/Default/6" /> |
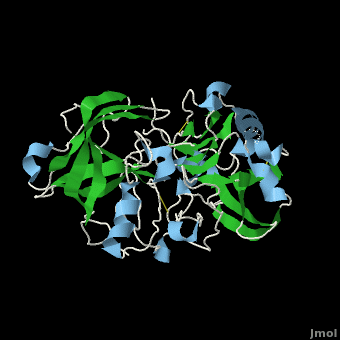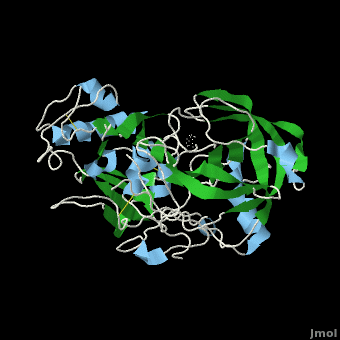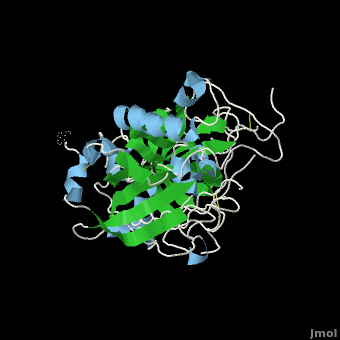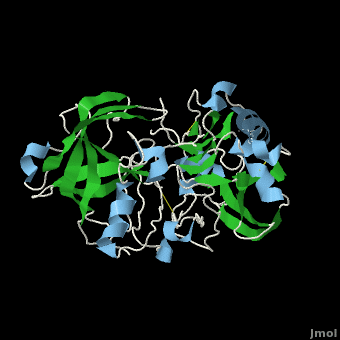
'''β-Secretase''', also known as '''BACE''' ('''β'''-site of '''A'''myloid precursor protein '''C'''leaving '''E'''nzyme) and '''Memapsin 2''', is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention. | '''β-Secretase''', also known as '''BACE''' ('''β'''-site of '''A'''myloid precursor protein '''C'''leaving '''E'''nzyme) and '''Memapsin 2''', is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention. | ||
| Line 6: | Line 6: | ||
==Structure of Beta Secretase== | ==Structure of Beta Secretase== | ||
| + | β-Secretase is a transmembrane protein, with its active site existing in the extracellular domain of the protein. Due to its nature as an aspartyl protease, β-secretase's active site is made up of <scene name='Beta_secretase/Activeasp3/2'>two aspartate residues: Asp32 and Asp228</scene>. The R groups of both aspartates coordinate a single water molecule between the two of them, allowing for a nucleophilic attack to occur on the carbonyls. | ||
| + | |||
| + | There are two other important features to β-secretase. One is the <scene name='Beta_secretase/Flap/1'>β hairpin loop over the active site, known as the "flap"</scene>. The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor. | ||
| + | |||
==Important Structures== | ==Important Structures== | ||
==Alzheimer's Disease== | ==Alzheimer's Disease== | ||
==Inhibition of Beta Secretase== | ==Inhibition of Beta Secretase== | ||
==References== | ==References== | ||
Revision as of 06:56, 30 November 2010
|
β-Secretase, also known as BACE (β-site of Amyloid precursor protein Cleaving Enzyme) and Memapsin 2, is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention.
Contents |
Structure of Beta Secretase
β-Secretase is a transmembrane protein, with its active site existing in the extracellular domain of the protein. Due to its nature as an aspartyl protease, β-secretase's active site is made up of . The R groups of both aspartates coordinate a single water molecule between the two of them, allowing for a nucleophilic attack to occur on the carbonyls.
There are two other important features to β-secretase. One is the . The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor.
Important Structures
Alzheimer's Disease
Inhibition of Beta Secretase
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Daniel Santos, Jaime Prilusky, Joel L. Sussman, David Canner