Beta secretase
From Proteopedia
| Line 1: | Line 1: | ||
<applet load="1w51" size="350" color="white" frame="true" align="right" caption="β-Secretase" scene="Beta_secretase/Default/6" /> | <applet load="1w51" size="350" color="white" frame="true" align="right" caption="β-Secretase" scene="Beta_secretase/Default/6" /> | ||
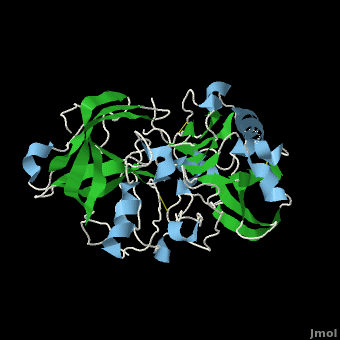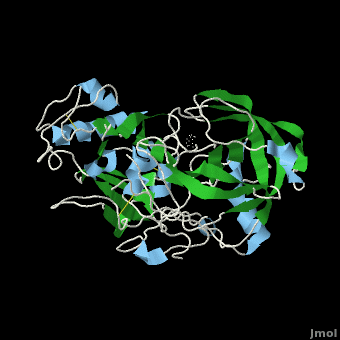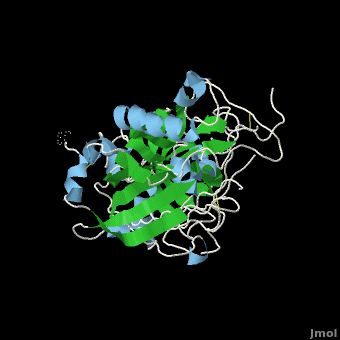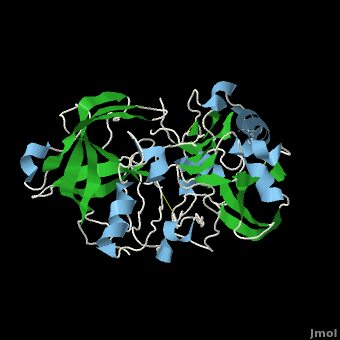
| - | <scene name='Beta_secretase/Default/6'> | + | <scene name='Beta_secretase/Default/6'>β-Secretase</scene>, also known as '''BACE''' ('''β'''-site of '''A'''myloid precursor protein '''C'''leaving '''E'''nzyme) and '''Memapsin 2''', is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention. |
__TOC__ | __TOC__ | ||
Revision as of 07:41, 30 November 2010
|
, also known as BACE (β-site of Amyloid precursor protein Cleaving Enzyme) and Memapsin 2, is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention.
Contents |
Structure of Beta Secretase
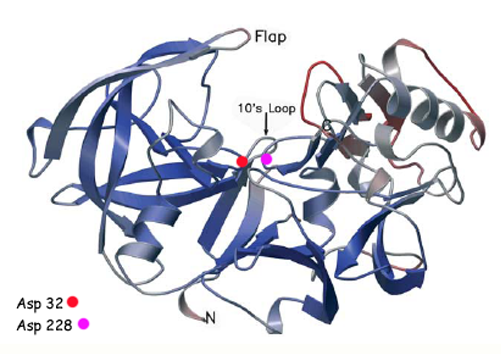
β-Secretase is a transmembrane protein, with its active site existing in the extracellular domain of the protein. Due to its nature as an aspartyl protease, β-secretase's active site is made up of . The R groups of both aspartates coordinate a single water molecule between the two of them, allowing for a nucleophilic attack to occur on the carbonyls.
There are two other important features to β-secretase. One is the . The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor.
The other important feature is the . The 10s loop is located in the S3 pocket of β-secretase, right between two β strands. When the 10s loop takes an open conformation, it allows for greater binding between the substrate and the S3 pocket. The 10s loop also contains within it a that can hydrogen bond with the substrate, allowing for further stabilization of the 10s loop, as well as the overall β-secretase-substrate interaction.
The three parts come together to form a sort of binding pocket for β-secretase's substrates or inhibitors. Binding to the active site activates the flap to close and initiates binding by the 10s loop, all to help stabilize the structure.
Alzheimer's Disease
Inhibition of Beta Secretase
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Daniel Santos, Jaime Prilusky, Joel L. Sussman, David Canner