Pore forming toxin, α-hemolysin
From Proteopedia
m (added topic page category) |
m |
||
| Line 13: | Line 13: | ||
==About this Structure== | ==About this Structure== | ||
| - | + | <table width=401' align='right' cellpadding='0'><tr><td rowspan='2'> </td><td bgcolor='#eeeeee'> | |
| + | <StructureSection load='7ahl' size='400' side='right' scene='7ahl/Davebriggscolor/4' caption='Hemolysin ([[7ahl]]), resolution 1.89Å. '> | ||
| + | ====The α-hemolysin oligomer==== | ||
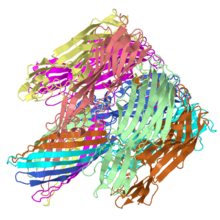
α-hemolysin ([[7ahl]]) is <scene name='7ahl/Davebriggscolor/4'>a 7 chain structure of protein sequences</scene> from [http://en.wikipedia.org/wiki/Staphylococcus_aureus Staphylococcus aureus]. | α-hemolysin ([[7ahl]]) is <scene name='7ahl/Davebriggscolor/4'>a 7 chain structure of protein sequences</scene> from [http://en.wikipedia.org/wiki/Staphylococcus_aureus Staphylococcus aureus]. | ||
| Line 39: | Line 41: | ||
====The α-hemolysin pore==== | ====The α-hemolysin pore==== | ||
| - | + | <nowiki>[</nowiki>Note: the following view generates a substantial surface which may take several minutes to calculate. Be patient.<nowiki>]</nowiki> Displaying the surface illustrates clearly that there is <scene name='7ahl/Davebriggscolorwithsurf/3'>a tunnel down the middle of the heptamer that leads to the formation of pores in the cell membrane</scene>, which is structural explanation for why these have . Such pores are expectedly detrimental to the cell, allowing exodus of critical molecules, destroying the established membrane potential and ionic gradients, and contributing to osmotic swelling. {{Link Toggle FancyCartoonHighQualityView}}. | |
| - | + | </StructureSection> | |
| - | + | </td></tr><tr><td ALIGN=RIGHT bgcolor='#ffffff'> | |
| - | + | <scene name='7ahl/Davebriggscolor/4'>Initial scene</scene> | |
| - | + | </td></tr></table> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==PDB entry== | ==PDB entry== | ||
Revision as of 05:17, 9 January 2011
α-Hemolysin from Staphlococcus aureus is a pore-forming toxin made of seven repeats of an identical monomer arranged in a ring. The structural basis of the toxic activity was readily revealed when the structure of the ring was solved.
Contents |
ALPHA-HEMOLYSIN FROM STAPHYLOCOCCUS AUREUS
The structure of the Staphylococcus aureus alpha-hemolysin pore has been determined to 1.9 A resolution. Contained within the mushroom-shaped homo-oligomeric heptamer is a solvent-filled channel, 100 A in length, that runs along the sevenfold axis and ranges from 14 A to 46 A in diameter. The lytic, transmembrane domain comprises the lower half of a 14-strand antiparallel beta barrel, to which each protomer contributes two beta strands, each 65 A long. The interior of the beta barrel is primarily hydrophilic, and the exterior has a hydrophobic belt 28 A wide. The structure proves the heptameric subunit stoichiometry of the alpha-hemolysin oligomer, shows that a glycine-rich and solvent-exposed region of a water-soluble protein can self-assemble to form a transmembrane pore of defined structure, and provides insight into the principles of membrane interaction and transport activity of beta barrel pore-forming toxins.
Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore., Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE, Science. 1996 Dec 13;274(5294):1859-66. PMID:8943190
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Background
This is just to show an example review citation[1].
About this Structure
| |||||||||||||
|
| |||||||||||||
PDB entry
7ahl is a 7 chain structure of sequences from Staphylococcus aureus. Full crystallographic information is available from OCA.
Reference for the structure
- Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996 Dec 13;274(5294):1859-66. PMID:8943190
Notes and Literature References
Additional Literature and Resources
For additional information, see: Toxins
David Briggs's Blog on α-hemolsyin, which served as a template for the original adaptation of 7ahl to this topic page.
Pore-forming toxin at Wikipedia
Page on Bacterial Toxin α-Hemolysin by Aleksei Aksimentiev and Klaus Schulten
Page started with original page seeded by OCA on Mon Feb 16 13:00:23 2009 for 7ahl