User:Caitlin Bell/Sandbox 1
From Proteopedia
| Line 15: | Line 15: | ||
The B subunit’s only function is to allow the cholera toxin to enter the intestinal epithelial cells through endocytosis. It accomplishes this from its unique structure. The B subunit contains five alpha helix proteins that are connected together to form a pentagon. Each alpha helix contains a single binding site for the intestinal lining, which is called its GM1 binding site (ganglioside binding site). | The B subunit’s only function is to allow the cholera toxin to enter the intestinal epithelial cells through endocytosis. It accomplishes this from its unique structure. The B subunit contains five alpha helix proteins that are connected together to form a pentagon. Each alpha helix contains a single binding site for the intestinal lining, which is called its GM1 binding site (ganglioside binding site). | ||
----- | ----- | ||
| - | ===Mechanism=== | + | ===Mechanism of Action=== |
----- | ----- | ||
Revision as of 14:29, 25 April 2011
Contents |
CHOLERA TOXIN
Introduction
Cholera toxin is released by the pathogen Vibrio cholerae during colonization of the small intestine. The genus Vibrio mainly corresponds with saltwater organisms, but there are some freshwater organisms. These organisms use glucose as their main energy and fuel source, and they use flagella for locomotion.
Cholera is widespread in mainly poverty-stricken areas where food and water environments are unsanitary. After ingestion of Vibrio cholerae, which typically is a result of feces particles in water or food, the cholera toxin is secreted and infects the small intestines, leading to the Cholera disease. Excessive diarrhea and vomiting ensues soon after ingestion, and death can occur within a few hours. Cholera is mainly prevalent in Africa, Asia, and Latin America, leading to 3-5 million cases and 100,000-200,000 deaths every year.
Structure
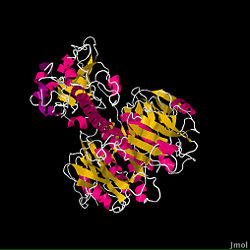
The fundamental structure of the cholera toxin is rather basic. It is a complex of six proteins that are structured into two subunits: A and B. The A subunit contains only one protein and is the only toxic part of the protein. The B subunit contains five proteins and is non-toxic.
The A subunit is the most destructive part of the cholera toxin. It can be further broken down into two chains: A1 and A2. These chains are held together through a single disulfide bond. The A1 chain is entirely non-polar, which allows it to pass through membranes. This is important for the cholera toxin’s function because it contains the fundamental enzymatic machinery. The A1 chain has a binding site for NAD+, which allows it to remove an ADP-ribose and transfer it to a G protein acceptor, which ultimately leads to the Cholera disease. On the other hand, the A2 chain’s main function is to connect the A1 chain to the B subunit.
The B subunit’s only function is to allow the cholera toxin to enter the intestinal epithelial cells through endocytosis. It accomplishes this from its unique structure. The B subunit contains five alpha helix proteins that are connected together to form a pentagon. Each alpha helix contains a single binding site for the intestinal lining, which is called its GM1 binding site (ganglioside binding site).