FOXP2
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
====Structure of Monomeric FOXP2==== | ====Structure of Monomeric FOXP2==== | ||
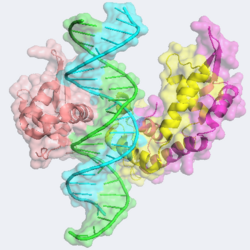
| - | The forkhead domain of FOXP2 assumes unique <scene name='FOXP2/Opening_dimer/1'>dimeric</scene> and <scene name='FOXP2/Opening_monomer/1'>monomeric</scene> structures bound to [[DNA]]. The monomeric form binds more tightly to DNA and assumes the canonical winged-helix motif present in all the FOX proteins. The <scene name='FOXP2/Opening_monomer_structure/2'>structure consists</scene> of four alpha helices capped by a two stranded beta sheet. As is present in all FOX proteins, the turn between helices 2 and 3 contains <scene name='FOXP2/3-10/1'>a 3-10 helix</scene>. DNA recognition of FOXP2 is mediated by <scene name='FOXP2/Opening_monomer_helix_3/1'>helix 3</scene>. Residues Asn 550, Arg 553, His 554, Ser 557, and Leu 558 form strong | + | The forkhead domain of FOXP2 assumes unique <scene name='FOXP2/Opening_dimer/1'>dimeric</scene> and <scene name='FOXP2/Opening_monomer/1'>monomeric</scene> structures bound to [[DNA]]. The monomeric form binds more tightly to DNA and assumes the canonical winged-helix motif present in all the FOX proteins. The <scene name='FOXP2/Opening_monomer_structure/2'>structure consists</scene> of four alpha helices capped by a two stranded beta sheet. As is present in all FOX proteins, the turn between helices 2 and 3 contains <scene name='FOXP2/3-10/1'>a 3-10 helix</scene>. DNA recognition of FOXP2 is mediated by <scene name='FOXP2/Opening_monomer_helix_3/1'>helix 3</scene>. Residues Asn 550, Arg 553, His 554, Ser 557, and Leu 558 form strong <scene name='FOXP2/H3_binding/1'>hydrogen bonding and hydrophobic interactions</scene> with the DNA. Additionally, residues Tyr 509, Leu 527, Trp 573 and Tyr 531 also <scene name='FOXP2/H3_binding_additoins/1'>interact from other helices</scene>, wedging helix H3 within the <scene name='FOXP2/Major_groove/1'>major groove of the DNA</scene>. Perhaps unsurprisingly, Arg 553 is **conserved** among all forkhead proteins. In fact, mutation of Arg 533 to a histidine has been linked to the severe congenital speech disorders mentioned previously. Also of note, mutation of Ile 363 to a valine results in reduced binding to DNA and IPEX syndrome a disease causing T-cell dysfunction and subsequent autoimmunity.<ref>PMID:18481161</ref><ref name="Chen">PMID:16407075</ref> |
====Structure of Dimeric FOXP2==== | ====Structure of Dimeric FOXP2==== | ||
Revision as of 18:37, 2 May 2011
| |||||||||||
References
- ↑ Pinker S. Talk of genetics and vice versa. Nature. 2001 Oct 4;413(6855):465-6. PMID:11586336 doi:10.1038/35097173
- ↑ Enard W, Gehre S, Hammerschmidt K, Holter SM, Blass T, Somel M, Bruckner MK, Schreiweis C, Winter C, Sohr R, Becker L, Wiebe V, Nickel B, Giger T, Muller U, Groszer M, Adler T, Aguilar A, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Favor J, Fuchs H, Gailus-Durner V, Hans W, Holzlwimmer G, Javaheri A, Kalaydjiev S, Kallnik M, Kling E, Kunder S, Mossbrugger I, Naton B, Racz I, Rathkolb B, Rozman J, Schrewe A, Busch DH, Graw J, Ivandic B, Klingenspor M, Klopstock T, Ollert M, Quintanilla-Martinez L, Schulz H, Wolf E, Wurst W, Zimmer A, Fisher SE, Morgenstern R, Arendt T, de Angelis MH, Fischer J, Schwarz J, Paabo S. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009 May 29;137(5):961-71. PMID:19490899 doi:10.1016/j.cell.2009.03.041
- ↑ Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol. 2008 Sep;28(5):581-7. Epub 2008 May 15. PMID:18481161 doi:10.1007/s10875-008-9196-1
- ↑ 4.0 4.1 Stroud JC, Wu Y, Bates DL, Han A, Nowick K, Paabo S, Tong H, Chen L. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006 Jan;14(1):159-66. PMID:16407075 doi:10.1016/j.str.2005.10.005
See Also
- Forkhead box family
- Forkhead box protein M1
- Transcription & RNA Processing
- Regulation of Gene Expression
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Wayne Decatur, Alexander Berchansky