Tom Sandbox
From Proteopedia
| Line 4: | Line 4: | ||
{{STRUCTURE_2zta| PDB=2zta | SCENE= }} | {{STRUCTURE_2zta| PDB=2zta | SCENE= }} | ||
| - | === | + | ===GCN4 - Leucine Zipper=== |
| - | |||
| - | <!-- | ||
| - | The line below this paragraph, {{ABSTRACT_PUBMED_1557122}}, adds the Publication Abstract to the page | ||
| - | (as it appears on PubMed at http://www.pubmed.gov), where 1557122 is the PubMed ID number. | ||
| - | --> | ||
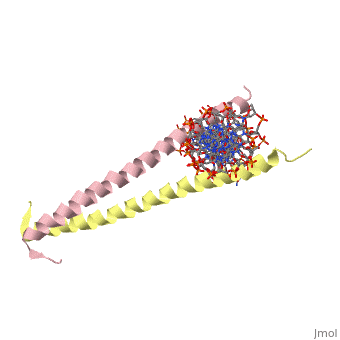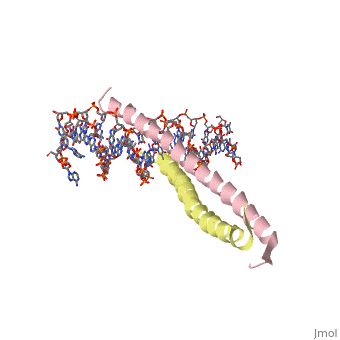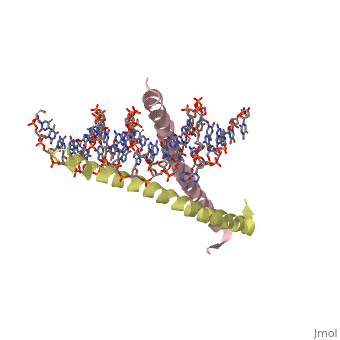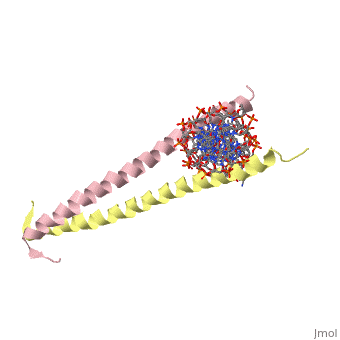
A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, <scene name='Tom_Sandbox/Cd_binding/1'>Zn(2+)-containing domain </scene>recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. | A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, <scene name='Tom_Sandbox/Cd_binding/1'>Zn(2+)-containing domain </scene>recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. | ||
| Line 16: | Line 11: | ||
==About this Structure== | ==About this Structure== | ||
| - | [[ | + | [[2zta]] is a 2 chain structure formed by |
| + | |||
==See Also== | ==See Also== | ||
Revision as of 00:25, 9 November 2011
| |||||||
| 2zta, resolution 1.80Å () | |||||||
|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Contents |
GCN4 - Leucine Zipper
A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4.
| |||||||||
| 1ysa, resolution 2.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
About this Structure
2zta is a 2 chain structure formed by
See Also
Reference
- Marmorstein R, Carey M, Ptashne M, Harrison SC. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122 doi:http://dx.doi.org/10.1038/356408a0