Tom Sandbox
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
[[Image:1ysa.png|left|300px]] | [[Image:1ysa.png|left|300px]] | ||
| + | ==GCN4 - The Leucine Zipper== | ||
{{STRUCTURE_2zta| PDB=2zta | SCENE= }} | {{STRUCTURE_2zta| PDB=2zta | SCENE= }} | ||
| - | ===GCN4 - Leucine Zipper=== | ||
Blah blah information about leucine zipper GCN4. | Blah blah information about leucine zipper GCN4. | ||
| Line 10: | Line 10: | ||
{{STRUCTURE_1ysa| PDB=1ysa | SCENE= }} | {{STRUCTURE_1ysa| PDB=1ysa | SCENE= }} | ||
| - | == | + | ===Structure=== |
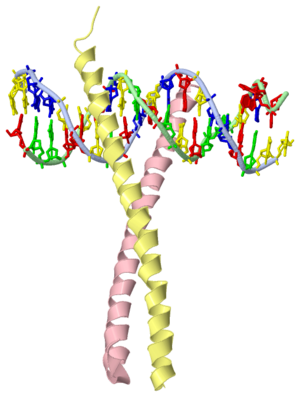
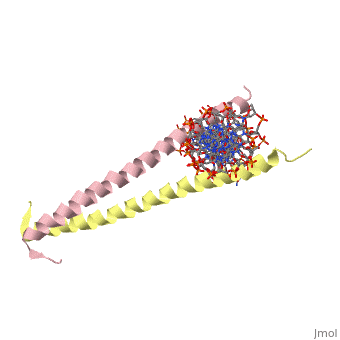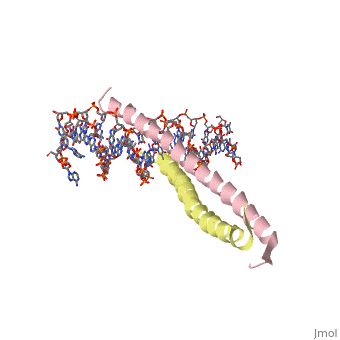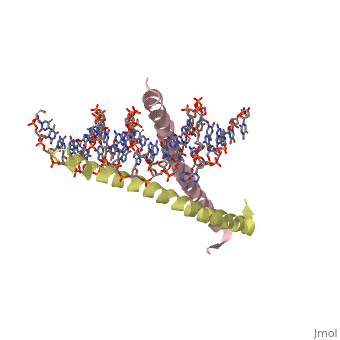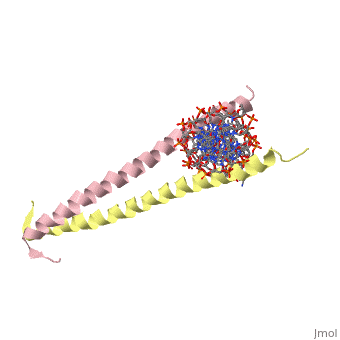
GCN4 (PDB [[2zta]] by itself, [[1ysa]] bound to DNA) is a eukaryotic transcription factor first isolated from yeast. It is composed of two identical 52 residue alpha helix chains that grouped together to form a dimer. The dimer binds through interlocking leucine amino acids in the C terminal ends, while pinching in on the major groove of DNA in the N terminal end. The X-ray structure of the 33-residue polypeptide corresponding to the leucine zipper of GCN4 was determined by Peter Kim and Thomas Alber<ref> Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008. </ref>. | GCN4 (PDB [[2zta]] by itself, [[1ysa]] bound to DNA) is a eukaryotic transcription factor first isolated from yeast. It is composed of two identical 52 residue alpha helix chains that grouped together to form a dimer. The dimer binds through interlocking leucine amino acids in the C terminal ends, while pinching in on the major groove of DNA in the N terminal end. The X-ray structure of the 33-residue polypeptide corresponding to the leucine zipper of GCN4 was determined by Peter Kim and Thomas Alber<ref> Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008. </ref>. | ||
| + | ===Binding=== | ||
| + | |||
| + | ====The Leucine Zipper==== | ||
| + | |||
| + | ====Binding with DNA==== | ||
==See Also== | ==See Also== | ||
Revision as of 00:56, 9 November 2011
Contents |
GCN4 - The Leucine Zipper
| |||||||
| 2zta, resolution 1.80Å () | |||||||
|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Blah blah information about leucine zipper GCN4.
| |||||||||
| 1ysa, resolution 2.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Structure
GCN4 (PDB 2zta by itself, 1ysa bound to DNA) is a eukaryotic transcription factor first isolated from yeast. It is composed of two identical 52 residue alpha helix chains that grouped together to form a dimer. The dimer binds through interlocking leucine amino acids in the C terminal ends, while pinching in on the major groove of DNA in the N terminal end. The X-ray structure of the 33-residue polypeptide corresponding to the leucine zipper of GCN4 was determined by Peter Kim and Thomas Alber[1].
Binding
The Leucine Zipper
Binding with DNA
See Also
Reference
- ↑ Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008.