This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Pertussis Toxin-ATP Complex
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
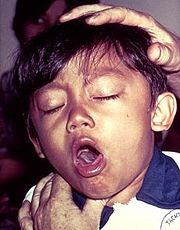
The second stage is the toxemic stage which follows the colonization stage. The PT B subunit oligomer uses cell-bound toxin (S2 and S3) as adhesins, and they bind the bacteria to host cells. S2 and S3 utilize different receptors on host cells. S2 binds specifically to a glycolipid, which is found primarily on the ciliated epithelial cells. S3 binds to a glycoprotein found mainly on phagocytic cells. After binding, the toxin is taken up by an endosome and transported from the plasma membrane via the Golgi apparatus to the endoplasmic reticulum (ER) where finally membrane translocation occurs. The destabilization of PT occurs in the ER prior to membrane translocation. After binding of ATP, cleavage of the single disulphide bond by [http://en.wikipedia.org/wiki/Protein_disulfide_isomerase protein disulphide isomerase] (PDI) occurs in subunit S1 <scene name='Pertussis_Toxin-ATP_Complex/Disulphide_bonds_breaking/1'>(Cys 41-Cys 201)</scene> and is believed to trigger a conformational change necessary to expose the active site to its substrates. The reducation step takes place after interaction of PT with ATP. | The second stage is the toxemic stage which follows the colonization stage. The PT B subunit oligomer uses cell-bound toxin (S2 and S3) as adhesins, and they bind the bacteria to host cells. S2 and S3 utilize different receptors on host cells. S2 binds specifically to a glycolipid, which is found primarily on the ciliated epithelial cells. S3 binds to a glycoprotein found mainly on phagocytic cells. After binding, the toxin is taken up by an endosome and transported from the plasma membrane via the Golgi apparatus to the endoplasmic reticulum (ER) where finally membrane translocation occurs. The destabilization of PT occurs in the ER prior to membrane translocation. After binding of ATP, cleavage of the single disulphide bond by [http://en.wikipedia.org/wiki/Protein_disulfide_isomerase protein disulphide isomerase] (PDI) occurs in subunit S1 <scene name='Pertussis_Toxin-ATP_Complex/Disulphide_bonds_breaking/1'>(Cys 41-Cys 201)</scene> and is believed to trigger a conformational change necessary to expose the active site to its substrates. The reducation step takes place after interaction of PT with ATP. | ||
| - | After destabilization, the S1 becomes active and catalyzes the [http://en.wikipedia.org/wiki/ADP_ribosylation ADP-ribosylation] of the alpa-subunit of regulatory trimeric [http://en.wikipedia.org/wiki/G_proteins G-proteins] (Giα) host protein. This then prevents Giα from inhibiting adenylate cyclase and leads to an increase in intracellular levels of [http://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate Cyclic adenosine monophosphate] (cAMP). The conversion of ATP to cyclic AMP cannot be stopped and intracellular levels of cAMP increase. | + | After destabilization, the S1 becomes active and catalyzes the [http://en.wikipedia.org/wiki/ADP_ribosylation ADP-ribosylation] of the alpa-subunit of regulatory trimeric [http://en.wikipedia.org/wiki/G_proteins G-proteins] (Giα) host protein. This then prevents Giα from inhibiting adenylate cyclase and leads to an increase in intracellular levels of [http://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate Cyclic adenosine monophosphate] (cAMP). The conversion of ATP to cyclic AMP cannot be stopped and intracellular levels of cAMP increase.<ref>Kenneth Todar, PhD. (2008)., http://www.textbookofbacteriology.net/pertussis.html</ref>This has the effect to disrupt cellular function/signaling, and in the case of phagocytes, to decrease their phagocytic activities such as chemotaxis, engulfment, the oxidative burst, and bacteridcidal killing.<ref>Kenneth Todar, PhD. (2008)., http://www.textbookofbacteriology.net/pertussis.html</ref> |
==Treatment== | ==Treatment== | ||
Revision as of 09:10, 12 November 2011
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Hazes B, Boodhoo A, Cockle SA, Read RJ. Crystal structure of the pertussis toxin-ATP complex: a molecular sensor. J Mol Biol. 1996 May 17;258(4):661-71. PMID:8637000 doi:10.1006/jmbi.1996.0277
- ↑ Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr Opin Pharmacol. 2007 Jun;7(3):272-8. Epub 2007 Apr 5. PMID:17418639 doi:10.1016/j.coph.2006.12.004
- ↑ Bettiol S, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, Harnden A. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003257. PMID:20091541 doi:10.1002/14651858.CD003257.pub3
- ↑ Weiss AA, Johnson FD, Burns DL. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2970-4. PMID:8464913
- ↑ Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992 Jun;6(9):2684-90. PMID:1612292
- ↑ Kenneth Todar, PhD. (2008)., http://www.textbookofbacteriology.net/pertussis.html
- ↑ Kenneth Todar, PhD. (2008)., http://www.textbookofbacteriology.net/pertussis.html
- ↑ Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007 Jul 18;(3):CD004404. PMID:17636756 doi:10.1002/14651858.CD004404.pub3