Journal:JBIC:16
From Proteopedia
(Difference between revisions)

| Line 11: | Line 11: | ||
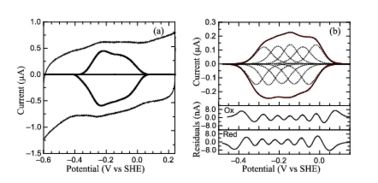
Protein film voltammetry (PFV) experiments performed on ''S. oneidensis'' ccNiR films in the absence of substrate produced a broad envelope of reversible signals that span approximately 450 mV. At high pH values the envelope appears as a single peak, whereas at pH values below 7 the envelope appears to be composed of two large overlapping peaks. At pH values below 6 and at 0 °C, the envelope of signal can be better resolved and more than two peaks can be observed. This resulting envelope of signal can be deconvoluted as the sum of five one-electron peaks, each corresponding to one of the five hemes in a ccNiR monomer (see image below). | Protein film voltammetry (PFV) experiments performed on ''S. oneidensis'' ccNiR films in the absence of substrate produced a broad envelope of reversible signals that span approximately 450 mV. At high pH values the envelope appears as a single peak, whereas at pH values below 7 the envelope appears to be composed of two large overlapping peaks. At pH values below 6 and at 0 °C, the envelope of signal can be better resolved and more than two peaks can be observed. This resulting envelope of signal can be deconvoluted as the sum of five one-electron peaks, each corresponding to one of the five hemes in a ccNiR monomer (see image below). | ||
[[Image:figur5.jpg|left|378px|thumb|PFV of ''S. oneidensis'' ccNiR (a) Typical signal on a graphite electrode. (b) Baselinesubtracted non-turnover voltammogram]] | [[Image:figur5.jpg|left|378px|thumb|PFV of ''S. oneidensis'' ccNiR (a) Typical signal on a graphite electrode. (b) Baselinesubtracted non-turnover voltammogram]] | ||
| - | The Ca<sup>2+</sup> ion within <scene name='Journal:JBIC:16/Cv/12'>conserved site</scene> is coordinated in bidentate fashion by Glu-205, and in monodentate fashion by the Tyr-206 and Lys-254 backbone carbonyls, and the Gln-256 side-chain carbonyl. In the ''S. oneidensis'' structure only one water molecule is assigned to the Ca<sup>2+</sup> ion in subunit B. In subunit A the difference electron density that represents this water molecule is very close to the noise level, and it is difficult to identify even one water molecule there. The carbonyl side chain of Asp-242 and the hydroxyl of Tyr-235 come near to the open calcium coordination sites, but are not within bonding distance. Instead they interact with the water molecule that is weakly coordinated to the Ca<sup>2+</sup> ion. The ccNiR calcium ions appear to play a vital role in organizing the <scene name='Journal:JBIC:16/Cv/13'>active site</scene> (as was mentioned above <font color='magenta'><b>hemes-1</b></font> are the active sites. | + | The Ca<sup>2+</sup> ion within <scene name='Journal:JBIC:16/Cv/12'>conserved site</scene> is coordinated in bidentate fashion by Glu-205, and in monodentate fashion by the Tyr-206 and Lys-254 backbone carbonyls, and the Gln-256 side-chain carbonyl. In the ''S. oneidensis'' structure only one water molecule is assigned to the Ca<sup>2+</sup> ion in subunit B. In subunit A the difference electron density that represents this water molecule is very close to the noise level, and it is difficult to identify even one water molecule there. The carbonyl side chain of Asp-242 and the hydroxyl of Tyr-235 come near to the open calcium coordination sites, but are not within bonding distance. Instead they interact with the water molecule that is weakly coordinated to the Ca<sup>2+</sup> ion. The ccNiR calcium ions appear to play a vital role in organizing the <scene name='Journal:JBIC:16/Cv/13'>active site</scene> (as was mentioned above <font color='magenta'><b>hemes-1</b></font> are the active sites). |
</StructureSection> | </StructureSection> | ||
<references/> | <references/> | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Revision as of 10:04, 4 March 2012
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.