1cfc
From Proteopedia
(New page: 200px<br /><applet load="1cfc" size="450" color="white" frame="true" align="right" spinBox="true" caption="1cfc" /> '''CALCIUM-FREE CALMODULIN'''<br /> ==Overview...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1cfc.gif|left|200px]]<br /><applet load="1cfc" size=" | + | [[Image:1cfc.gif|left|200px]]<br /><applet load="1cfc" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1cfc" /> | caption="1cfc" /> | ||
'''CALCIUM-FREE CALMODULIN'''<br /> | '''CALCIUM-FREE CALMODULIN'''<br /> | ||
==Overview== | ==Overview== | ||
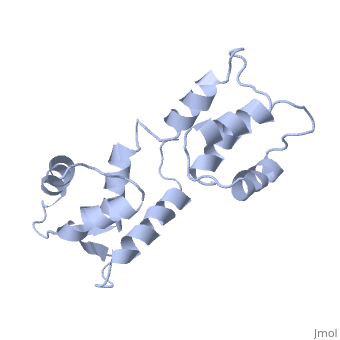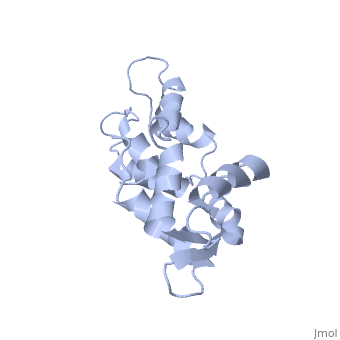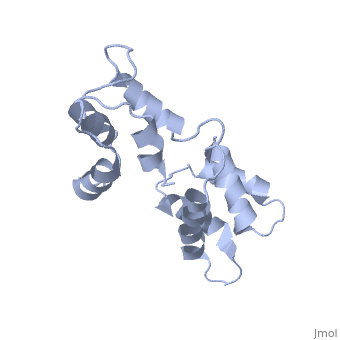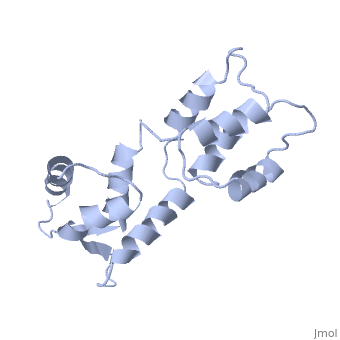
| - | The three-dimensional structure of calmodulin in the absence of Ca2+ has | + | The three-dimensional structure of calmodulin in the absence of Ca2+ has been determined by three- and four-dimensional heteronuclear NMR experiments, including ROE, isotope-filtering combined with reverse labelling, and measurement of more than 700 three-bond J-couplings. In analogy with the Ca(2+)-ligated state of this protein, it consists of two small globular domains separated by a flexible linker, with no stable, direct contacts between the two domains. In the absence of Ca2+, the four helices in each of the two globular domains form a highly twisted bundle, capped by a short anti-parallel beta-sheet. This arrangement is qualitatively similar to that observed in the crystal structure of the Ca(2+)-free N-terminal domain of troponin C. |
==About this Structure== | ==About this Structure== | ||
| - | 1CFC is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Xenopus_laevis Xenopus laevis]. Full crystallographic information is available from [http:// | + | 1CFC is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Xenopus_laevis Xenopus laevis]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CFC OCA]. |
==Reference== | ==Reference== | ||
| Line 15: | Line 15: | ||
[[Category: Bax, A.]] | [[Category: Bax, A.]] | ||
[[Category: Grzesiek, S.]] | [[Category: Grzesiek, S.]] | ||
| - | [[Category: Klee, C | + | [[Category: Klee, C B.]] |
[[Category: Kuboniwa, H.]] | [[Category: Kuboniwa, H.]] | ||
[[Category: Ren, H.]] | [[Category: Ren, H.]] | ||
| Line 21: | Line 21: | ||
[[Category: calcium-binding protein]] | [[Category: calcium-binding protein]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 12:05:32 2008'' |
Revision as of 10:05, 21 February 2008
|
CALCIUM-FREE CALMODULIN
Overview
The three-dimensional structure of calmodulin in the absence of Ca2+ has been determined by three- and four-dimensional heteronuclear NMR experiments, including ROE, isotope-filtering combined with reverse labelling, and measurement of more than 700 three-bond J-couplings. In analogy with the Ca(2+)-ligated state of this protein, it consists of two small globular domains separated by a flexible linker, with no stable, direct contacts between the two domains. In the absence of Ca2+, the four helices in each of the two globular domains form a highly twisted bundle, capped by a short anti-parallel beta-sheet. This arrangement is qualitatively similar to that observed in the crystal structure of the Ca(2+)-free N-terminal domain of troponin C.
About this Structure
1CFC is a Single protein structure of sequence from Xenopus laevis. Full crystallographic information is available from OCA.
Reference
Solution structure of calcium-free calmodulin., Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A, Nat Struct Biol. 1995 Sep;2(9):768-76. PMID:7552748
Page seeded by OCA on Thu Feb 21 12:05:32 2008