Staphylococcal nuclease
From Proteopedia
| Line 4: | Line 4: | ||
==Structure== | ==Structure== | ||
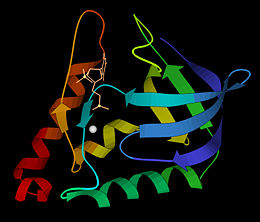
The first structure was solved in 1969 in the Cotton lab at MIT, published in 1971<ref>Arnone AA, Bier CJ, Cotton FA, Day VW, Hazen EE Jr, Richardson DC, Richardson JS, Yonath A (1971) "A High Resolution Structure of an Inhibitor Complex of the Extracellular Nuclease of Staphylococcus aureus: I. Experimental Procedures and Chain Tracing," J. Biol. Chem. 246, 2303-2316</ref>, and deposited in the PDB as now-obsoleted 1sns in April 1974. The highest resolution wildtype structure in the PDB with deposited data is 2snc at 1.65A[http://www.rcsb.org/pdb/explore/explore.do?structureId=1SNC]. As seen in the ribbon diagram above, the nuclease molecule has 3 long alpha helices and a 5-stranded, barrel-shaped beta sheet, in an arrangement known as the OB-fold (for oligonucleotide-binding fold) as classified in the SCOP database. There are about 200 PDB entries for Staph. nuclease, including extremely extensive mutational studies from many labs focused on understanding aspects of protein folding<ref>PubMed:22496593</ref><ref>PubMed: 15228960</ref><ref>PubMed: 8762134</ref>. | The first structure was solved in 1969 in the Cotton lab at MIT, published in 1971<ref>Arnone AA, Bier CJ, Cotton FA, Day VW, Hazen EE Jr, Richardson DC, Richardson JS, Yonath A (1971) "A High Resolution Structure of an Inhibitor Complex of the Extracellular Nuclease of Staphylococcus aureus: I. Experimental Procedures and Chain Tracing," J. Biol. Chem. 246, 2303-2316</ref>, and deposited in the PDB as now-obsoleted 1sns in April 1974. The highest resolution wildtype structure in the PDB with deposited data is 2snc at 1.65A[http://www.rcsb.org/pdb/explore/explore.do?structureId=1SNC]. As seen in the ribbon diagram above, the nuclease molecule has 3 long alpha helices and a 5-stranded, barrel-shaped beta sheet, in an arrangement known as the OB-fold (for oligonucleotide-binding fold) as classified in the SCOP database. There are about 200 PDB entries for Staph. nuclease, including extremely extensive mutational studies from many labs focused on understanding aspects of protein folding<ref>PubMed:22496593</ref><ref>PubMed: 15228960</ref><ref>PubMed: 8762134</ref>. | ||
| + | |||
| + | ==3D structures of Staphylococcal nuclease== | ||
| + | |||
| + | [[1sno – SCN – ''Staphylococcus aureus''<br /> | ||
| + | [[1tr5]], [[1u9r]], [[2pw5]], [[2pyk]], [[2pzw – SCN (mutant) – Room temperature | ||
| + | [[3sk4]], [[3sk5]], [[3sk6]], [[3sk8]], [[3sxh]], [[3t13]], [[3hej]], [[3hzx]], [[3nhh]], [[3itp]], [[3p75]], [[3lx0]], [[3meh]], [[3mvv]], [[3qoj]], [[3qol]], [[3qon]], [[3mhb]], [[3mxp]], [[3mz5]], [[3r3o]], [[3ruz]], [[3t16]], [[3tme]], [[3tp5]], [[3tp6]], [[3tp7]], [[3tp8]], [[3v2t]], [[3qb3]], [[3va5]], [[4df7]], [[4dfa]], [[4dgz]], [[4du9]], [[4e6i]], [[4eoa]], [[4eqn]], [[4eqo]], [[4eqp]], [[4evo]], [[4f7x]], [[4f8m]], [[2pw7]], [[2pzt]], [[2pzu]], [[2rdf]], [[2qdb]], [[2rbm]], [[3bdc]], [[3c1e]], [[3c1f]], [[3d4d]], [[3d6c]], [[3dmu]], [[3e5s]], [[3eji]], [[3ero]], [[3erq]], [[3d4w]], [[3d8g]], [[3dhq]], [[3h6m]], [[2f0e]], [[2f0f]], [[2f0g]], [[2f0h]], [[2f0i]], [[2f0j]], [[2f0k]], [[2f0l]], [[2f0m]], [[2f0n]], [[2f0o]], [[2f0p]], [[2f0q]], [[2f0s]], [[2f0t]], [[2f0u]], [[2f0v]], [[2f0w]], [[2oxp]], [[2oeo]], [[2of1]], [[2rks]], [[1snp]], [[1snq]], [[1joo]], [[1jor]], [[1tqo]], [[1tt2]], [[2exz]], [[2ey1]], [[2ey2]], [[2ey5]], [[2ey6]], [[2eyf]], [[2eyh]], [[2eyj]], [[2eyl]], [[2eym]], [[2eyo]], [[2eyp]], [[2f0d – SCN (mutant) – Low temperature<br /> | ||
| + | [[2khs – SCN - NMR<BR /> | ||
| + | [[2f3v – SCN residues 1-110 (mutant) – NMR<BR /> | ||
| + | [[2pqe – SCN residues 80-228 (mutant) – NMR<BR /> | ||
| + | [[2f3w – SCN residues 1-110 – NMR<BR /> | ||
| + | [[1jok]], [[1joq]], [[3s9w]], [[3sr1 - SCN (mutant) + Ca + TBP | ||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 09:27, 8 August 2012
Micrococcal nuclease, or Staphylococcal nuclease, is a monomeric Ca++ dependent enzyme of 149 amino acids that cleaves either DNA or RNA substrates. It is used for relatively non-specific cleavage of nucleic acids in molecular biology, and has been an important model system for the study of protein folding.
|
Structure
The first structure was solved in 1969 in the Cotton lab at MIT, published in 1971[1], and deposited in the PDB as now-obsoleted 1sns in April 1974. The highest resolution wildtype structure in the PDB with deposited data is 2snc at 1.65A[1]. As seen in the ribbon diagram above, the nuclease molecule has 3 long alpha helices and a 5-stranded, barrel-shaped beta sheet, in an arrangement known as the OB-fold (for oligonucleotide-binding fold) as classified in the SCOP database. There are about 200 PDB entries for Staph. nuclease, including extremely extensive mutational studies from many labs focused on understanding aspects of protein folding[2][3][4].
3D structures of Staphylococcal nuclease
[[1sno – SCN – Staphylococcus aureus
1tr5, 1u9r, 2pw5, 2pyk, [[2pzw – SCN (mutant) – Room temperature
3sk4, 3sk5, 3sk6, 3sk8, 3sxh, 3t13, 3hej, 3hzx, 3nhh, 3itp, 3p75, 3lx0, 3meh, 3mvv, 3qoj, 3qol, 3qon, 3mhb, 3mxp, 3mz5, 3r3o, 3ruz, 3t16, 3tme, 3tp5, 3tp6, 3tp7, 3tp8, 3v2t, 3qb3, 3va5, 4df7, 4dfa, 4dgz, 4du9, 4e6i, 4eoa, 4eqn, 4eqo, 4eqp, 4evo, 4f7x, 4f8m, 2pw7, 2pzt, 2pzu, 2rdf, 2qdb, 2rbm, 3bdc, 3c1e, 3c1f, 3d4d, 3d6c, 3dmu, 3e5s, 3eji, 3ero, 3erq, 3d4w, 3d8g, 3dhq, 3h6m, 2f0e, 2f0f, 2f0g, 2f0h, 2f0i, 2f0j, 2f0k, 2f0l, 2f0m, 2f0n, 2f0o, 2f0p, 2f0q, 2f0s, 2f0t, 2f0u, 2f0v, 2f0w, 2oxp, 2oeo, 2of1, 2rks, 1snp, 1snq, 1joo, 1jor, 1tqo, 1tt2, 2exz, 2ey1, 2ey2, 2ey5, 2ey6, 2eyf, 2eyh, 2eyj, 2eyl, 2eym, 2eyo, 2eyp, [[2f0d – SCN (mutant) – Low temperature
[[2khs – SCN - NMR
[[2f3v – SCN residues 1-110 (mutant) – NMR
[[2pqe – SCN residues 80-228 (mutant) – NMR
[[2f3w – SCN residues 1-110 – NMR
1jok, 1joq, 3s9w, [[3sr1 - SCN (mutant) + Ca + TBP
References
- ↑ Arnone AA, Bier CJ, Cotton FA, Day VW, Hazen EE Jr, Richardson DC, Richardson JS, Yonath A (1971) "A High Resolution Structure of an Inhibitor Complex of the Extracellular Nuclease of Staphylococcus aureus: I. Experimental Procedures and Chain Tracing," J. Biol. Chem. 246, 2303-2316
- ↑ PubMed:22496593
- ↑ PubMed: 15228960
- ↑ PubMed: 8762134
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky, Jane S. Richardson