1io1
From Proteopedia
(New page: 200px<br /><applet load="1io1" size="450" color="white" frame="true" align="right" spinBox="true" caption="1io1, resolution 2.0Å" /> '''CRYSTAL STRUCTURE OF ...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1io1.gif|left|200px]]<br /><applet load="1io1" size=" | + | [[Image:1io1.gif|left|200px]]<br /><applet load="1io1" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1io1, resolution 2.0Å" /> | caption="1io1, resolution 2.0Å" /> | ||
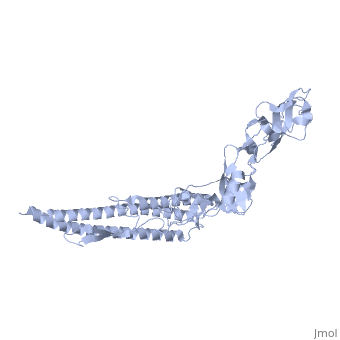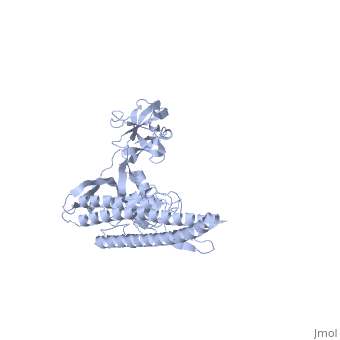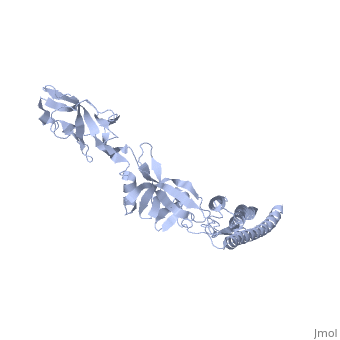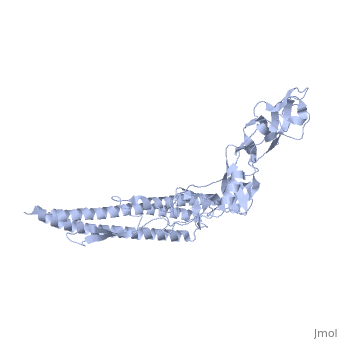
'''CRYSTAL STRUCTURE OF F41 FRAGMENT OF FLAGELLIN'''<br /> | '''CRYSTAL STRUCTURE OF F41 FRAGMENT OF FLAGELLIN'''<br /> | ||
==Overview== | ==Overview== | ||
| - | The bacterial flagellar filament is a helical propeller constructed from | + | The bacterial flagellar filament is a helical propeller constructed from 11 protofilaments of a single protein, flagellin. The filament switches between left- and right-handed supercoiled forms when bacteria switch their swimming mode between running and tumbling. Supercoiling is produced by two different packing interactions of flagellin called L and R. In switching from L to R, the intersubunit distance ( approximately 52 A) along the protofilament decreases by 0.8 A. Changes in the number of L and R protofilaments govern supercoiling of the filament. Here we report the 2.0 A resolution crystal structure of a Salmonella flagellin fragment of relative molecular mass 41,300. The crystal contains pairs of antiparallel straight protofilaments with the R-type repeat. By simulated extension of the protofilament model, we have identified possible switch regions responsible for the bi-stable mechanical switch that generates the 0.8 A difference in repeat distance. |
==About this Structure== | ==About this Structure== | ||
| - | 1IO1 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]. Full crystallographic information is available from [http:// | + | 1IO1 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1IO1 OCA]. |
==Reference== | ==Reference== | ||
| Line 17: | Line 17: | ||
[[Category: Nagashima, S.]] | [[Category: Nagashima, S.]] | ||
[[Category: Namba, K.]] | [[Category: Namba, K.]] | ||
| - | [[Category: Samatey, F | + | [[Category: Samatey, F A.]] |
[[Category: Vondervisz, F.]] | [[Category: Vondervisz, F.]] | ||
[[Category: Yamamoto, M.]] | [[Category: Yamamoto, M.]] | ||
| Line 23: | Line 23: | ||
[[Category: flagellin]] | [[Category: flagellin]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 13:13:45 2008'' |
Revision as of 11:13, 21 February 2008
|
CRYSTAL STRUCTURE OF F41 FRAGMENT OF FLAGELLIN
Overview
The bacterial flagellar filament is a helical propeller constructed from 11 protofilaments of a single protein, flagellin. The filament switches between left- and right-handed supercoiled forms when bacteria switch their swimming mode between running and tumbling. Supercoiling is produced by two different packing interactions of flagellin called L and R. In switching from L to R, the intersubunit distance ( approximately 52 A) along the protofilament decreases by 0.8 A. Changes in the number of L and R protofilaments govern supercoiling of the filament. Here we report the 2.0 A resolution crystal structure of a Salmonella flagellin fragment of relative molecular mass 41,300. The crystal contains pairs of antiparallel straight protofilaments with the R-type repeat. By simulated extension of the protofilament model, we have identified possible switch regions responsible for the bi-stable mechanical switch that generates the 0.8 A difference in repeat distance.
About this Structure
1IO1 is a Single protein structure of sequence from Salmonella typhimurium. Full crystallographic information is available from OCA.
Reference
Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling., Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K, Nature. 2001 Mar 15;410(6826):331-7. PMID:11268201
Page seeded by OCA on Thu Feb 21 13:13:45 2008