Group:MUZIC:Telethonin
From Proteopedia

| Line 49: | Line 49: | ||
Another interaction has been reported, and also associated with pathology, the one with bone morphogenetic protein-10 (BMP10). The interaction of telethonin with BMP10 is described as a sensor of increased wall stress of the left ventricle. A BMP10 variant is associated with hypertension dilated cardiomyopathy; its binding to telethonin is reduced, and its extracellular secretion is increased, causing cardiomyocyte hypertrophy. <ref>PMID:17921333 </ref> | Another interaction has been reported, and also associated with pathology, the one with bone morphogenetic protein-10 (BMP10). The interaction of telethonin with BMP10 is described as a sensor of increased wall stress of the left ventricle. A BMP10 variant is associated with hypertension dilated cardiomyopathy; its binding to telethonin is reduced, and its extracellular secretion is increased, causing cardiomyocyte hypertrophy. <ref>PMID:17921333 </ref> | ||
| + | |||
| + | |||
| + | [http://http://www.uniprot.org/uniprot/O15273 human], [http://www.uniprot.org/uniprot/O70548 mouse], [http://www.uniprot.org/uniprot/Q6T8D8 bovine], [http://www.uniprot.org/uniprot/A4GR69 porcine] | ||
Revision as of 07:44, 25 October 2012
Telethonin
Also known as T-Cap or Titin Cap protein. It is a small protein of 19kDa, 167 amino acids. It is predominantly expressed in striated muscle. It is a structural protein of the muscle; it is associated to the Z-disc in the sarcomere. It acts as multifunctional protein linking titin and other proteins implicated in sarcomere structure and signalling pathways.
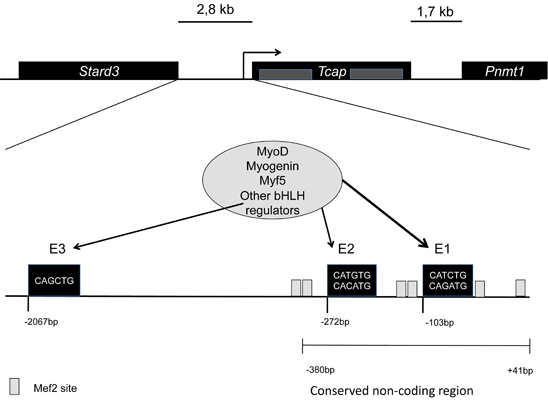
Gene regulation and expressionIt is encoded by the Tcap gene, in mice (Mus musculus) and TCAP in humans (Homo sapiens). In mice it is located in chromosome 11, in humans in the long arm of chromosome 17. Tcap is encoded by two exons, and has non-conserved intragenic sequences. The gene is flanked by two other genes, namely Stard3 upstream separated by 2,8kb, and Pnmt1 downstream separated by 1,7kb. It has three conserved E-box elements at -103bp (E1), -272bp (E2), and -2067bp (E3). For the full activation of the gene the regulation of E1 is highly important. MyoD plays an important role in this regulation all through development, while myogenin mainly during late differentiation into myoblasts. [1]
At the transcriptional level it is thought that there is only one isoform of Tcap, and it is one of the most abundant transcripts in skeletal muscle [2]. It does not have different levels of expression in different types of fibers in skeletal muscle; levels of expression of Tcap are lower in neonatal compared to adult striated muscle. The transcript is accumulated in a linear pattern similar to that of the myosin heavy chain [3]. In these same studies it was reported that denervation leads to decrease in the expression of Tcap, suggesting that locomotor activity is a potential regulator of its maintenance.
Tcap gene product, the protein TelethoninTelethonin protein is found mostly in skeletal and cardiac muscle. It is one of the major components of the sarcomere, it is localized to the Z-disc. It was also reported a nuclear localization.[4], [5] Studies on telethonin structure by Zou et al. [6] report that it is made up of (N-terminal in blue and C-ter in orange) The structure of telethonin was determined using X-ray crystallography. [7],[6] The shape and architecture of the complex of titin/telethonin was studied by small-angle- X-ray scattering (SAXS) and then compared to the crystallographic models. They also used in-vitro experiments to follow the formation of the complex in non-myogenic Cos1 cells, in order to understand if the assemblage is possible [8] This symmetry of telethonin permits its interaction with titin. Both are assembled in an antiparallel (titin:telethonin). Titin N-terminal domains Z1 and Z2 (two Ig like repeats) interact with the C-terminal region of telethonin (residues 1-53). Telethonin mediates in the antiparallel assembly of the two Z1Z2domains. In early differentiating myocytes titin C-terminal and telethonin co-localize and titin kinase is close to telethonin C-terminal, and it is phosphorylated. This phosphorylation is involved in the reorganization of the cytoskeleton during myofibrillogenesis. [9] This co-localization is not seen in adult myofibrils, titin kinase is reported to localize in the M-band [9]; It was also informed that telethonin interacts with other proteins including: Potassium channel β-subunit of the slow activating component of the delayed rectifier potassium current (IKs) channel (minK) [10], ankyrin1, and Z-disc proteins FATZ,/Myozenin-1/ Calsarcin-3 [11], and Ankrd2.[12] Telethonin interacts with minK’s cytoplasmic domain. MinK binds specifically to the sixteen C-terminal residues of telethonin. This suggest that minK, telethonin ant titin form a complex that links myofibrils to the sarcolemma. Phosphorilation of telethonin in Ser157 is a negative regulation for this interaction. This interaction occurs in cardiac myofibrils, it has been reported that minK is not expressed in skeletal muscle. [10] Telethonin interacts with FATZ/Myozenin-1/Calsarcin-3 N-terminal between residues 78-125. It might be an association as mechanosensing and stretch-associated signalling machinery. [11] The interaction between Ankrd2 and telethonin has been proposed as a sensor of muscle stress/stretch and a starting point for the transmission of the mechanical signal to the nucleus regulating gene expression. [12] Telethonin is also involved in signalling processes that regulate muscle development. A feed back loop is formed with MRFs (MyoD, myogenin, Myf5) regulating Tcap gene expression; telethonin interacts with myostatin, inhibiting it. So it regulates MyoD through the Myostatin – Smad3 pathway. [13]. There is an interaction with MDM2 N-terminal. MDM2 is capable of redirecting telethonin to the nucleus. Telethonin is inhibited by MDM2 in a dose dependent manner. In cells MDM2 is involved in the regulation of proteasomal turnover of telethonin. [5] Another interaction has been reported, and also associated with pathology, the one with bone morphogenetic protein-10 (BMP10). The interaction of telethonin with BMP10 is described as a sensor of increased wall stress of the left ventricle. A BMP10 variant is associated with hypertension dilated cardiomyopathy; its binding to telethonin is reduced, and its extracellular secretion is increased, causing cardiomyocyte hypertrophy. [14]
Pathologies associated with telethoninDifferent mutations in telethonin have been associated with several myopathies. Mutations can lead to limb-girdle muscular dystrophy type 2G (LGMD2G) [15], to hypertrophic cardiopathy, [16] and dilated cardiomyopathy. Two mutations found in the Tcap gene cause a deletion of the telethonin C-terminal region, losing the site which can be phosphorylated, for example by titin kinase [15], leading to disruption of the sarcomeric structure; as was observed in a few brazilian families with LGMD2G. Defects in the MLP-Tcap association are linked to human dilated cardiomyopathy and heart failure (Knöll 2002). Mutations that affect ability of MLP to interact with telethonin result in the loss of telethonin binding, facilitating its mislocalization from the complex with titin, leading to defects in the Z-disc and progression of dilated cardiomyopathy. Knöll et al. conclude that genetic mutations causing a incorrect interaction of telethonin with MLP can lead to a development of human dilated cardiomyopathy through modifications in the conformation and function of titin. [16] It was reported that in 10 cases of neurogenic atrophy there was a decreased staining for telethonin in type II fibers, and in early stages of fiber atrophy, [17] indicating a selective downregulation of telethonin. These observations can be related to in vivo studies done in rats, in which after short term denervation (two days), Tcap transcript is reduced by about 50% in skeletal muscle. [3].
References
| ||||||||||||