User:Josie N. Harmon/Sandbox 1
From Proteopedia
< User:Josie N. Harmon(Difference between revisions)
| (23 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == '''Xanthine Oxidase in | + | == '''Xanthine Oxidase''' == |
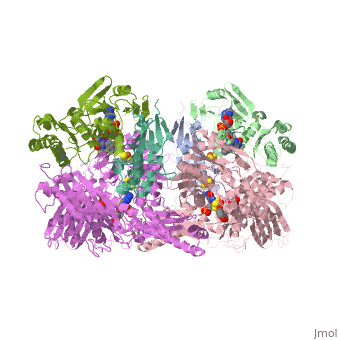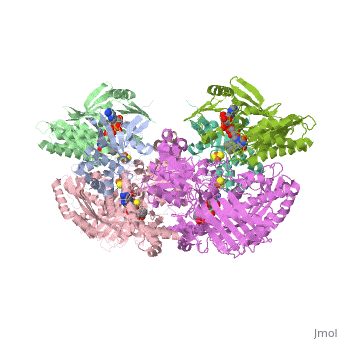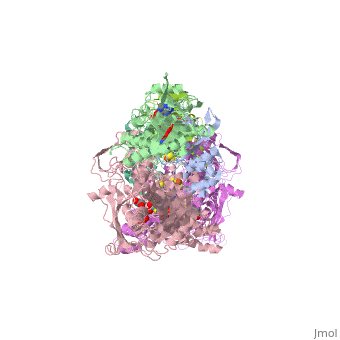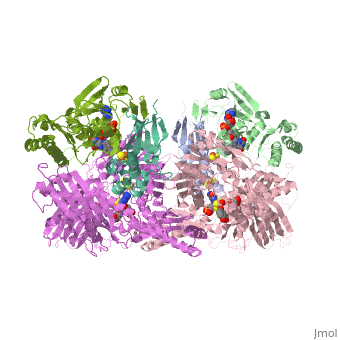
| + | <StructureSection load='1fiq' size='500' side='right' caption='Crystal structure of Xanthine Oxidase from Bovine Milk (PDB entry [[1FIQ]])' scene=''>Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. In eukaryotes xanthine oxidases exist as homodimers with each <scene name='User:Josie_N._Harmon/Sandbox_1/Monomer_ligands/3'>monomer</scene> containing <scene name='User:Josie_N._Harmon/Sandbox_1/Ligands_monomer/4'>four redox-active sites.</scene> The crystalline structure of a xanthine oxidase monomer offers a better view of the <scene name='User:Josie_N._Harmon/Sandbox_1/Mos_mte_name/1'>active molybdenum center</scene>, the ferredoxin iron sulfur, <scene name='User:Josie_N._Harmon/Sandbox_1/Fe2s2_name/1'>Fe2S2,</scene> clusters, and <scene name='User:Josie_N._Harmon/Sandbox_1/Fad_name/1'>FAD</scene>. | ||
| - | + | == '''Xanthine Oxidoreductase''' == | |
| - | + | Xanthine oxidase is considered a component of <scene name='User:Josie_N._Harmon/Sandbox_1/Xanthine_oxidoreductase/2'>xanthine oxidoreductase</scene> along with xanthine dehydrogenase, which is an enzyme known to generate reactive oxygen species. This enzyme is considered to be extremely important in the catabolism of purines in several steps to yield <scene name='User:Josie_N._Harmon/Sandbox_1/Uric_acid/1'>uric acid</scene> which is ultimately excreted from the body, through a series of oxidation steps than involve metabolizing hypoxanthine to xanthine, which is further oxidized to uric acid. The xanthine oxidoreductase enzyme can exists in two different states, one being the xanthine oxidase conformer and the other being the xanthine dehydrogenase conformer. There are several disulfide bridges within the oxidoreductase enzyme and if these bridges are left intact the enzyme acts as an oxidase, but if these bridges are cleaved the enzyme acts as a dehydrogenase. Also the oxidoreductase enzyme can be permanently cleaved by proteases so that it always acts in the oxidase form. One side of the xanthine oxidoreductase enzyme consists of an <scene name='User:Josie_N._Harmon/Sandbox_1/Catalytic_site/2'>active site </scene>that includes a molybdenum atom which binds to a purine substrate and adds a hydroxyl group. During this process electrons are extracted and funneled from the active site through a string of iron-sulfur clusters to the opposing side of the enzyme. The opposing side then transfers the electrons to NAD or oxygen depending on the dehydrogenase or oxidase nature of the enzyme. One of the final steps in the electron transfer funnels electrons to a FAD group. The dehydrogenase form of the enzyme transfers these electrons to NAD, while the oxidase form blocks NAD through a loop of protein that covers the FAD molecule allowing smaller oxygen molecules to accept the electrons. A <scene name='User:Josie_N._Harmon/Sandbox_1/6mercaptopurine_ligand/3'>secondary depiction</scene> of the structure is represented with purple arrows representing the alpha helix structures and gold arrows representing the beta strand structures. The <scene name='User:Josie_N._Harmon/Sandbox_1/Mercap_helix_blue/1'>helices </scene>of the protein backbone can also be noted by the blue arrows on the structure. The xanthine oxidase complex consists of numerous interactions such as <scene name='User:Josie_N._Harmon/Sandbox_1/Fad_h_bond/2'>hydrogen bonding</scene>, pi-pi interactions, and hydrophobic interactions. | |
| - | 6-Mercaptopurine | + | == '''6-Mercaptopurine''' == |
| + | <scene name='User:Josie_N._Harmon/Sandbox_1/6_mp_name/1'>6-Mercaptopurine</scene> is metabolized hepatically by xanthine oxidase. 6-Mercaptopurine is classified as a cytotoxic chemotherapy agent frequently used to treat acute lymphoblastic leukemia (ALL), non-Hodgkin's lymphoma, psoriatic arthritis, and inflammatory bowel disease. The drug belongs to a class of compounds referred to as purine antagonists that inhhibits DNA and RNA synthesis. The drug acts as a antimetabolite and incorporates itself into DNA or RNA and virtually inhibits the growth of cancer cells. The drug allopurinol, which is used to treat gout, is very similar in structure to 6-mercaptopurine and it is this similarity that makes the use of the two drugs simultaneously contraindicated. Allopurinol acts by inhibiting xanthine oxidase, the enzyme that metabolizes 6-mercaptopurine. | ||
| - | 6-Mercaptopurine is classified as a cytotoxic chemotherapy agent frequently used to treat acute lymphoblastic leukemia (ALL), non-Hodgkin's lymphoma, psoriatic arthritis, and inflammatory bowel disease. The drug belongs to a class of compounds referred to as purine antagonists that inhhibits DNA and RNA synthesis. The drug acts as a antimetabolite and incorporates itself into DNA or RNA and virtually inhibits the growth of cancer cells. The drug allopurinol, which is used to treat gout, is very similar in structure to 6-mercaptopurine and it is this similarity that makes the use of the two drugs simultaneously contraindicated. Allopurinol acts by inhibiting xanthine oxidase, the enzyme that metabolizes 6-mercaptopurine. | ||
| - | + | == '''Xanthine Oxidase Inhibitors''' == | |
| + | Xanthine oxidase inhibitors act by inhibiting the activity of the enzyme, namely its purine metabolism activity. Inhibitors of the enzyme are commonly used in the treatment of hyperuricemia, and its corresponding medical conditions such as gout, by reducing the production of uric acid. Currently there is also investigation of the use of xanthine oxidase inhibitors in the prevention and treatment of cardiovascular and cerebrovascular disease. As previously mentioned xanthine oxidase plays an important role in purine degradation with the last step in this process resulting in the production of uric acid to be excreted from the body. This excretion; however, is not always an efficient process and there can be an abnormal accumulation of uric acid in the blood. This accumulation can come as a result of increased production by the way of a purine rich diet, decreased excretion by the way of drug interactions or genetics, or a combination of the two. The most common type of xanthine oxidase inhibitors are classified as purine analogues and consists of allopurinol and oxypurinol.</StructureSection> | ||
Current revision
Xanthine Oxidase
| |||||||||||