We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Tutorial:Basic Chemistry Topics
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=='''Summary: Scientific Research Artical'''== | =='''Summary: Scientific Research Artical'''== | ||
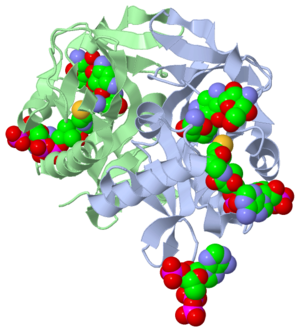
| - | *The study where this molecule was obtained is named "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin". The study focused on AAC (2’)- Ic, also known as aminoglycoside 2’- N- acetyltransferase. The scientists in the study determined the crystal structure of AAC (2’)-Ic from Mycobacterium tuberculosis. The specific fold of AAC (2’)-Ic is placed in the GNAT or GCN5-related N-acetyltransferase superfamily. Although the physiological function of AAC(2’)-Ic is not certain, the crystal structure determined by the scientists allowed them to hypothesize. Through the crystal structure, scientists determined that this enzyme might acetylate mycothiol, which is a key biosynthetic intermediate and the major reducing agent in mycobacterium. This enzyme is capable of acetylating aminoglycosides bearing a 2’ amino group. When this occurs the aminoglycoside antibiotic becomes inactive.<references | + | *The study where this molecule was obtained is named "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin". The study focused on AAC (2’)- Ic, also known as aminoglycoside 2’- N- acetyltransferase. The scientists in the study determined the crystal structure of AAC (2’)-Ic from Mycobacterium tuberculosis. The specific fold of AAC (2’)-Ic is placed in the GNAT or GCN5-related N-acetyltransferase superfamily. Although the physiological function of AAC(2’)-Ic is not certain, the crystal structure determined by the scientists allowed them to hypothesize. Through the crystal structure, scientists determined that this enzyme might acetylate mycothiol, which is a key biosynthetic intermediate and the major reducing agent in mycobacterium. This enzyme is capable of acetylating aminoglycosides bearing a 2’ amino group. When this occurs the aminoglycoside antibiotic becomes inactive.<references group="1"/> |
| Line 45: | Line 45: | ||
==Ionic Bonds== | ==Ionic Bonds== | ||
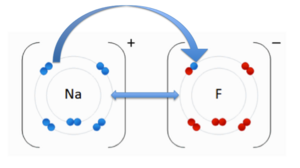
| - | [[Image:Ionic bond.png| thumb | right | 300px | Ionic Bonding<ref | + | [[Image:Ionic bond.png| thumb | right | 300px | Ionic Bonding<ref group="1">. "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.</ref>]] |
An ionic bond is an attraction between two molecules of opposite charge. The opposite charges are positive (+) and negative (-). A positively charged atom is referred to as a cation, and a negatively charged atom is referred to as an anion. To the image to the right, you can see a negatively charged Fluorine (F) and the cation Sodium (Na). These two atoms are attracted to each other due to there opposite charges. The double-sided arrow between them is representation of there attractive force. | An ionic bond is an attraction between two molecules of opposite charge. The opposite charges are positive (+) and negative (-). A positively charged atom is referred to as a cation, and a negatively charged atom is referred to as an anion. To the image to the right, you can see a negatively charged Fluorine (F) and the cation Sodium (Na). These two atoms are attracted to each other due to there opposite charges. The double-sided arrow between them is representation of there attractive force. | ||
In this representation the pink depicts the negatively charged (anionic/acidic) portion of the molecule and the yellow represents the positively charged (cationic/basic) portion of the molecule. Through this representation you will notice that the charges are evenly distributed. They are evenly distributed because the positive and negative charges are attracted to one and other, while the positive-positive and negative-negative charges repel each other. The repulsion of common charges and the attraction of oppositely charged atoms/molecules is what causes them to distribute evenly. <scene name='Tutorial:Basic_Chemistry_Topics/Ionic_bond/3'>positive and negative charges</scene> | In this representation the pink depicts the negatively charged (anionic/acidic) portion of the molecule and the yellow represents the positively charged (cationic/basic) portion of the molecule. Through this representation you will notice that the charges are evenly distributed. They are evenly distributed because the positive and negative charges are attracted to one and other, while the positive-positive and negative-negative charges repel each other. The repulsion of common charges and the attraction of oppositely charged atoms/molecules is what causes them to distribute evenly. <scene name='Tutorial:Basic_Chemistry_Topics/Ionic_bond/3'>positive and negative charges</scene> | ||
| Line 51: | Line 51: | ||
==Hydrogen Bonds== | ==Hydrogen Bonds== | ||
| - | [[Image:3D hydrogen Bonding.jpg | thumb | left | 200px | Hydrogen Bonding<ref | + | [[Image:3D hydrogen Bonding.jpg | thumb | left | 200px | Hydrogen Bonding<ref group="2">Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.</ref> |
Hydrogen Bonds, the weakest of bonds, are attractive interactions between an electronegative atom and hydrogen. Electronegative atoms are atoms that have high electron density. They are strong atoms that pull electrons towards them from weaker/low electron density atoms, such as hydrogen. When the electronegative atom pulls the electrons, it leaves the other atom with a slightly positive charge. Water is the most common example of hydrogen bonding. The water molecule chemical formula is H2O. The highly electronegative oxygen pulls the hydrogen closer by attracting hydrogen’s electrons and allowing the formation of a water droplet. The electronegative atoms allow for the droplet to be held together instead of spreading. In this representation the hydrogen bonds are represented as yellow-dashed lines. The hydrogen bonds are important to the stability of the secondary structures. <scene name='Tutorial:Basic_Chemistry_Topics/Hydrogen_bonds/2'>Hydrogen Bonds</scene> | Hydrogen Bonds, the weakest of bonds, are attractive interactions between an electronegative atom and hydrogen. Electronegative atoms are atoms that have high electron density. They are strong atoms that pull electrons towards them from weaker/low electron density atoms, such as hydrogen. When the electronegative atom pulls the electrons, it leaves the other atom with a slightly positive charge. Water is the most common example of hydrogen bonding. The water molecule chemical formula is H2O. The highly electronegative oxygen pulls the hydrogen closer by attracting hydrogen’s electrons and allowing the formation of a water droplet. The electronegative atoms allow for the droplet to be held together instead of spreading. In this representation the hydrogen bonds are represented as yellow-dashed lines. The hydrogen bonds are important to the stability of the secondary structures. <scene name='Tutorial:Basic_Chemistry_Topics/Hydrogen_bonds/2'>Hydrogen Bonds</scene> | ||
Revision as of 23:07, 31 October 2012
| |||||||||||