Josie N. Harmon/Sandbox Tutorial
From Proteopedia
| Line 3: | Line 3: | ||
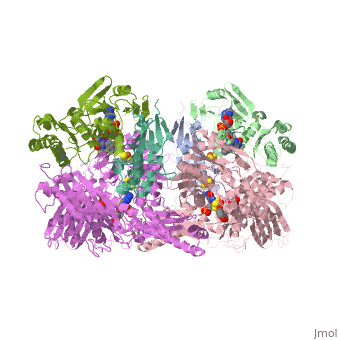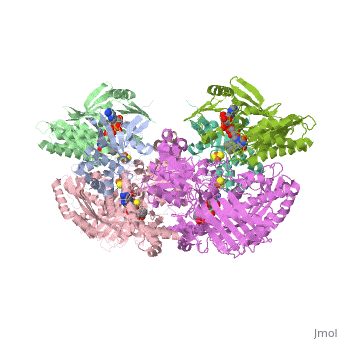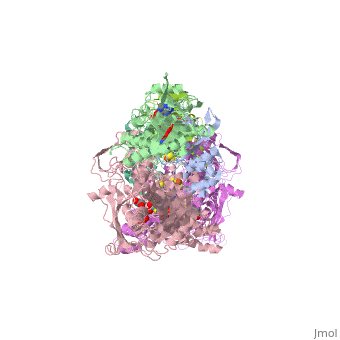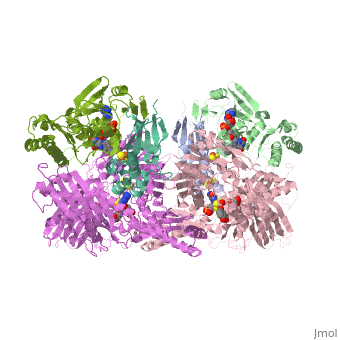
<StructureSection load='1fiq' size='350' side='right' caption='Crystal Structure of Xanthine Oxidase from Bovine Milk (PDB entry [[1fiq]])' scene=''> | <StructureSection load='1fiq' size='350' side='right' caption='Crystal Structure of Xanthine Oxidase from Bovine Milk (PDB entry [[1fiq]])' scene=''> | ||
| - | == Xanthine | + | == Xanthine Oxidoreductase == |
Xanthine oxidoreductase is actualy considered to exists as two enzymes in one, one being xanthine oxidase and the other existing as xanthine dehydrogenase. When the enzyme was initially purified by scientists two different forms of the enzyme were found, where one uses nicotinamide adenine dinucleotide (NAD) and the other uses oxygen. The two forms were believed to be two different enzymes and were thus given two different names; however, upon further investigation into the amino acid sequence of the enzymes it was determined that the enzymes were actually the same. The two forms of the enzyme can differ in two ways: | Xanthine oxidoreductase is actualy considered to exists as two enzymes in one, one being xanthine oxidase and the other existing as xanthine dehydrogenase. When the enzyme was initially purified by scientists two different forms of the enzyme were found, where one uses nicotinamide adenine dinucleotide (NAD) and the other uses oxygen. The two forms were believed to be two different enzymes and were thus given two different names; however, upon further investigation into the amino acid sequence of the enzymes it was determined that the enzymes were actually the same. The two forms of the enzyme can differ in two ways: | ||
| Line 15: | Line 15: | ||
| - | == Mechanism of | + | == Mechanism of Action == |
Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. In eukaryotes xanthine oxidases exist as homodimers with each monomer containing four redox-active sites. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, Fe2S2, clusters, and FAD. | Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. In eukaryotes xanthine oxidases exist as homodimers with each monomer containing four redox-active sites. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, Fe2S2, clusters, and FAD. | ||
| Line 21: | Line 21: | ||
[[Image:urate_reaction.jpg]] | [[Image:urate_reaction.jpg]] | ||
| - | == Clinical | + | == Clinical Application == |
| - | == Xanthine | + | == Xanthine Oxidase Inhibitors == |
Xanthine oxidase inhibitors act by inhibiting the activity of the enzyme, namely its purine metabolism activity. Inhibitors of the enzyme are commonly used in the treatment of hyperuricemia, and its corresponding medical conditions such as gout, by reducing the production of uric acid. Currently there is also investigation of the use of xanthine oxidase inhibitors in the prevention and treatment of cardiovascular and cerebrovascular disease. As previously mentioned xanthine oxidase plays an important role in purine degradation with the last step in this process resulting in the production of uric acid to be excreted from the body. This excretion; however, is not always an efficient process and there can be an abnormal accumulation of uric acid in the blood. This accumulation can come as a result of increased production by the way of a purine rich diet, decreased excretion by the way of drug interactions or genetics, or a combination of the two. The most common type of xanthine oxidase inhibitors are classified as purine analogues and consists of allopurinol and oxypurinol. | Xanthine oxidase inhibitors act by inhibiting the activity of the enzyme, namely its purine metabolism activity. Inhibitors of the enzyme are commonly used in the treatment of hyperuricemia, and its corresponding medical conditions such as gout, by reducing the production of uric acid. Currently there is also investigation of the use of xanthine oxidase inhibitors in the prevention and treatment of cardiovascular and cerebrovascular disease. As previously mentioned xanthine oxidase plays an important role in purine degradation with the last step in this process resulting in the production of uric acid to be excreted from the body. This excretion; however, is not always an efficient process and there can be an abnormal accumulation of uric acid in the blood. This accumulation can come as a result of increased production by the way of a purine rich diet, decreased excretion by the way of drug interactions or genetics, or a combination of the two. The most common type of xanthine oxidase inhibitors are classified as purine analogues and consists of allopurinol and oxypurinol. | ||
Revision as of 19:17, 7 November 2012
Xanthine Oxidase Biochemistry Tutorial
The human diet introduces a large assortment of various new molecules into the body. These molecules are often degraded and used by the body to be later utilized as a source of metabolic energy. In other cirmcumstances the molecules can be broken down into components to be used by the body to build necessary proteins and nucleic acids. Lastly, any molecules that are remaining following the previous processes can be degraded for elimination. Xanthine oxidoreductase is considered to be the final stop for extra purine nucleotides, such as adenosine triphosphate (ATP)Image:ATP.pngand guanosine triphosphate (GTP)Image:GTP.png, in our cells. Inside the cells the enzyme takes on a role of purine degredation, where it is involved in the extrememly inportant catabolism of purines through a series of steps to yield uric acid which is ultimately excreted from the body.
| |||||||||||