Catalytic Subunit of T. Castaneum TERT Polymerase
From Proteopedia
| Line 1: | Line 1: | ||
| - | =Overview= | ||
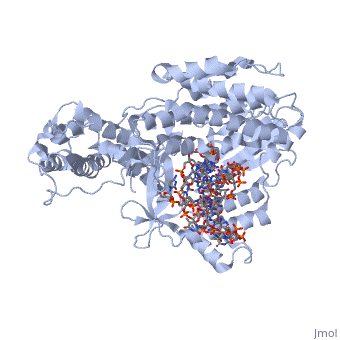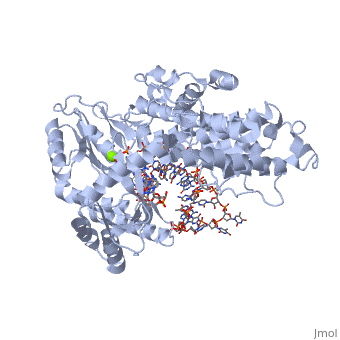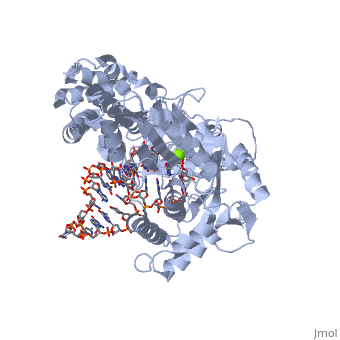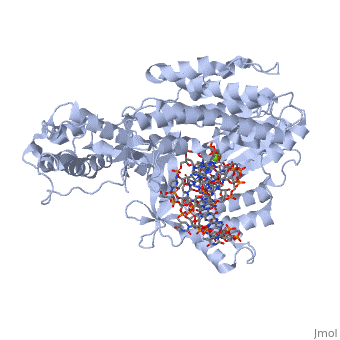
<Structure load='3kyl' size='500' frame='true' align='right' caption='Catalytic Subunit of TERT Polymerase bound to RNA Promoter and DNA Template Strand' scene='Insert optional scene name here' /> | <Structure load='3kyl' size='500' frame='true' align='right' caption='Catalytic Subunit of TERT Polymerase bound to RNA Promoter and DNA Template Strand' scene='Insert optional scene name here' /> | ||
| - | Telomerase is a specialized DNA polymerase that extends the 3' ends of eukaryotic linear chromosomes, a process required for genomic stability and cell viability. Here we present the crystal structure of the active Tribolium castaneum telomerase catalytic subunit, TERT, bound to an RNA-DNA hairpin designed to resemble the putative RNA-templating region and telomeric DNA. The RNA-DNA hybrid adopts a helical structure, docked in the interior cavity of the TERT ring. Contacts between the RNA template and motifs 2 and B' position the solvent-accessible RNA bases close to the enzyme active site for nucleotide binding and selectivity. Nucleic acid binding induces rigid TERT conformational changes to form a tight catalytic complex. Overall, TERT-RNA template and TERT-telomeric DNA associations are remarkably similar to those observed for retroviral reverse transcriptases, suggesting common mechanistic aspects of DNA replication between the two families of enzymes. | ||
| - | ---- | + | =Abstract= |
| + | |||
| + | Telomerase is a specialized DNA polymerase that extends the 3' ends of eukaryotic linear chromosomes, a process required for genomic stability and cell viability. Here we present the crystal structure of the active Tribolium castaneum telomerase catalytic subunit, TERT, bound to an RNA-DNA hairpin designed to resemble the putative RNA-templating region and telomeric DNA. The RNA-DNA hybrid adopts a helical structure, docked in the interior cavity of the TERT ring. Contacts between the RNA template and motifs 2 and B' position the solvent-accessible RNA bases close to the enzyme active site for nucleotide binding and selectivity. Nucleic acid binding induces rigid TERT conformational changes to form a tight catalytic complex. Overall, TERT-RNA template and TERT-telomeric DNA associations are remarkably similar to those observed for retroviral reverse transcriptases, suggesting common mechanistic aspects of DNA replication between the two families of enzymes. | ||
| - | ==DNA Grip Region== | ||
<scene name='Catalytic_Subunit_of_T._Castaneum_TERT_Polymerase/Asp_residues_hold_dna_in_place/2'>DNA Grip Region</scene> | <scene name='Catalytic_Subunit_of_T._Castaneum_TERT_Polymerase/Asp_residues_hold_dna_in_place/2'>DNA Grip Region</scene> | ||
| Line 15: | Line 14: | ||
<scene name='Catalytic_Subunit_of_T._Castaneum_TERT_Polymerase/Val_342/1'>Active Site of the Enzyme</scene> | <scene name='Catalytic_Subunit_of_T._Castaneum_TERT_Polymerase/Val_342/1'>Active Site of the Enzyme</scene> | ||
| + | |||
| + | Highly positively charged pocket: | ||
| + | |||
| + | |||
| + | =Differences Between Human Telomerase= | ||
| + | |||
| + | </StructureSection> | ||
Revision as of 06:04, 2 December 2012
|
Abstract
Telomerase is a specialized DNA polymerase that extends the 3' ends of eukaryotic linear chromosomes, a process required for genomic stability and cell viability. Here we present the crystal structure of the active Tribolium castaneum telomerase catalytic subunit, TERT, bound to an RNA-DNA hairpin designed to resemble the putative RNA-templating region and telomeric DNA. The RNA-DNA hybrid adopts a helical structure, docked in the interior cavity of the TERT ring. Contacts between the RNA template and motifs 2 and B' position the solvent-accessible RNA bases close to the enzyme active site for nucleotide binding and selectivity. Nucleic acid binding induces rigid TERT conformational changes to form a tight catalytic complex. Overall, TERT-RNA template and TERT-telomeric DNA associations are remarkably similar to those observed for retroviral reverse transcriptases, suggesting common mechanistic aspects of DNA replication between the two families of enzymes.
Highly positively charged pocket:
Differences Between Human Telomerase
</StructureSection>