Tutorial:Basic Chemistry Topics
From Proteopedia
| (One intermediate revision not shown.) | |||
| Line 260: | Line 260: | ||
='''Secondary Structures'''= | ='''Secondary Structures'''= | ||
| - | Secondary structures are <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_beta_2ndstructures/1'>alpha helices and beta sheets</scene>. The helices and sheets provide stability to the compound as a whole. The alpha helices are represented with pink arrows and the beta sheets are represented with yellow arrows. This molecule has approximately eight alpha helices and four beta sheets. <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_helix/1'>Alpha Helices </scene>have a cylinder-like structure with a parallel formation. In this representation you can see the parallel formation within the cylinder structure. The parallel alpha helices are held in its cylinder structure by hydrogen bonds. <scene name='Tutorial:Basic_Chemistry_Topics/Beta_sheets/1'>Beta sheets</scene> are often anti-parallel. The folding of a protein, alpha helices and beta sheets, gives the compound its function. When there is a change in protein folding, the function will change. As previously stated, the study discovered ACC(2’)-Ic to have a GNAT fold, and the GNAT family are enzymes capable of acetylation. <"Wikipedia. N.p., n.d. Web. 12 Nov. 2012. <http://en.wikipedia.org/wiki/Protein_secondary_structure>. </ref> | + | Secondary structures are <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_beta_2ndstructures/1'>alpha helices and beta sheets</scene>. The helices and sheets provide stability to the compound as a whole. The alpha helices are represented with pink arrows and the beta sheets are represented with yellow arrows. This molecule has approximately eight alpha helices and four beta sheets. <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_helix/1'>Alpha Helices </scene>have a cylinder-like structure with a parallel formation. In this representation you can see the parallel formation within the cylinder structure. The parallel alpha helices are held in its cylinder structure by hydrogen bonds. <scene name='Tutorial:Basic_Chemistry_Topics/Beta_sheets/1'>Beta sheets</scene> are often anti-parallel. The folding of a protein, alpha helices and beta sheets, gives the compound its function. When there is a change in protein folding, the function will change. As previously stated, the study discovered ACC(2’)-Ic to have a GNAT fold, and the GNAT family are enzymes capable of acetylation. <ref> "Wikipedia. N.p., n.d. Web. 12 Nov. 2012. <http://en.wikipedia.org/wiki/Protein_secondary_structure>. </ref> |
| Line 291: | Line 291: | ||
! scope="col" width="5000px" | Coenzyme A (CoA) | ! scope="col" width="5000px" | Coenzyme A (CoA) | ||
|- | |- | ||
| - | | scope="col" width="5000px" | Coenzyme A (CoA) is involved in many physiological processes such as, synthesizing and oxidizing fatty acids. This process is essential for the utilization of fatty acids for energy. Coenzyme A is used as a substrate in the citric acid cycle. The citric acid cycle is also known as the Krebs cycle or tricarboxylic acid cycle (TCA). This process is important to the production of ATP, which is an energy source used by the body. | + | | scope="col" width="5000px" | Coenzyme A (CoA) is involved in many physiological processes such as, synthesizing and oxidizing fatty acids. This process is essential for the utilization of fatty acids for energy. Coenzyme A is used as a substrate in the citric acid cycle. The citric acid cycle is also known as the Krebs cycle or tricarboxylic acid cycle (TCA). This process is important to the production of ATP, which is an energy source used by the body.<ref>Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." "Coenzyme A." Wikipedia. N.p., n.d. Web. 11 Nov. 2012. <http://en.wikipedia.org/wiki/Coenzyme_A>. </ref> |
|} | |} | ||
| Line 298: | Line 298: | ||
|- | |- | ||
| scope="col" width="5000px" | | | scope="col" width="5000px" | | ||
| - | Tobramycin is an antibiotic which is part of the aminoglycoside family. Aminoglycosides produce antibacterial effects by inhibiting protein synthesis and compromising the structure of the cell wall. By inhibiting protein synthesis of bacteria it prevents the bacteria from replicating. The cell wall is an important structure to bacteria, because it provides the structure and stability to the bacteria. By disrupting the cell wall, the stability of the bacteria is destroyed and ultimately causing bacteria cell death. | + | Tobramycin is an antibiotic which is part of the aminoglycoside family. Aminoglycosides produce antibacterial effects by inhibiting protein synthesis and compromising the structure of the cell wall. By inhibiting protein synthesis of bacteria it prevents the bacteria from replicating. The cell wall is an important structure to bacteria, because it provides the structure and stability to the bacteria. By disrupting the cell wall, the stability of the bacteria is destroyed and ultimately causing bacteria cell death.<ref name="Tobramycin"/> |
| - | Tobramycin targets a variety of bacteria, particularly gram(-) species. Just like all drugs there are side effects associated with tobramycin. Some of the more common side effects are ototoxicity and nephrotoxicity. Ototoxicity is hearing loss and nephrotoxicity is kidney damage. The kidney damage is due to tobramycin reabsorption through the renal tubules. This basically means that tobramycin may be toxic to the kidneys due to prolonged contact time in the kidneys. | + | Tobramycin targets a variety of bacteria, particularly gram(-) species. Just like all drugs there are side effects associated with tobramycin. Some of the more common side effects are ototoxicity and nephrotoxicity. Ototoxicity is hearing loss and nephrotoxicity is kidney damage. The kidney damage is due to tobramycin reabsorption through the renal tubules. This basically means that tobramycin may be toxic to the kidneys due to prolonged contact time in the kidneys.<ref name="Tobramycin"/> |
| - | Tobramycin is a pregnancy category D. Pregnancy categories are assigned to all drugs. They are used to classify how likely the drug is to cause harm to the fetus. The pregnancy categories are A, B, C, D, and X. Pregnancy category A causes no harm to the fetus and pregnancy category X definitely causes harm to the fetus. Since Tobramycin is a pregnancy category D, this is not an optimal choice for a pregnant patient with a gram(-) bacterial infection. | + | Tobramycin is a pregnancy category D. Pregnancy categories are assigned to all drugs. They are used to classify how likely the drug is to cause harm to the fetus. The pregnancy categories are A, B, C, D, and X. Pregnancy category A causes no harm to the fetus and pregnancy category X definitely causes harm to the fetus. Since Tobramycin is a pregnancy category D, this is not an optimal choice for a pregnant patient with a gram(-) bacterial infection.<ref name="Tobramycin"/> |
Tobramycin can be given intravenously, intramuscularly, as an inhalation or ophthalmically. Intravenously (IV) is a route of administration where the drug is administered directly to the vasculature or blood vessels. Intramuscular (IM) injection penetrates through the skin to the muscle. A common example of an intramuscular injection is a flu shot. An inhalation delivers the drug directly into the lungs. An example of inhalation drug administration is an inhaler used for asthmatics. Ophthalmic administration is drug administration directly to the eye, such as an eye drop. <ref name="Tobramycin">"Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.</ref> | Tobramycin can be given intravenously, intramuscularly, as an inhalation or ophthalmically. Intravenously (IV) is a route of administration where the drug is administered directly to the vasculature or blood vessels. Intramuscular (IM) injection penetrates through the skin to the muscle. A common example of an intramuscular injection is a flu shot. An inhalation delivers the drug directly into the lungs. An example of inhalation drug administration is an inhaler used for asthmatics. Ophthalmic administration is drug administration directly to the eye, such as an eye drop. <ref name="Tobramycin">"Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.</ref> | ||
Current revision
Purpose of the Tutorial
- This tutorial is intended as a beneficial learning/teaching aid for an entry-level chemistry college student with some basic chemistry knowledge. Various general chemistry concepts are explained using a research article as an example. Applying general chemistry to a research article will allow the students to see the impact they can have on the research world in the future by applying their knowledge.
Summary: Scientific Research Article
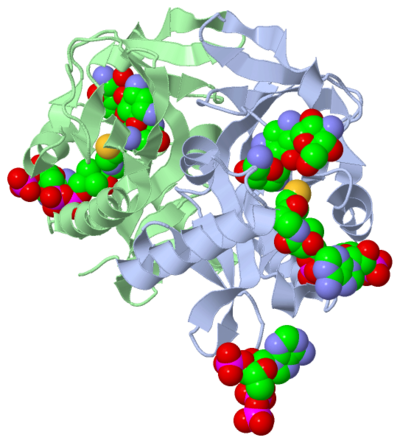
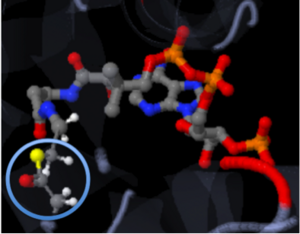
The molecule to left is from the article "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin" published in Nature Structural Biology.[2]. The study focused on aminoglycoside 2’- N- acetyltransferase (AAC (2’)- Ic), an enzyme. This enzyme is a protein that speeds the rate of the reaction it catalyzes.
This study determined the structure of AAC (2’)-Ic from mycobacterium tuberculosis, a pathogen. This pathogen is a microorganism that causes tuberculosis (TB), which typically affects the lungs, but can affect other parts of the body as well. The specific structure/protein fold of AAC (2’)-Ic places it in the GCN5-related N-acetyltransferase (GNAT) superfamily. The GNAT superfamily is a group of enzymes that are similar in structure. The protein fold is important because it determines the function of the compound.[2]
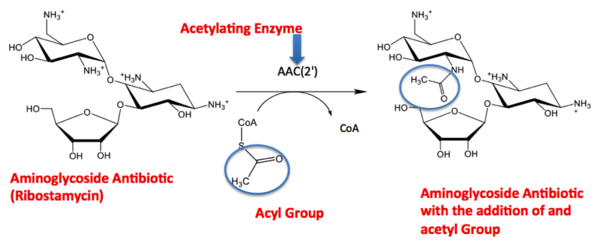
The GNAT family is a group of acetylating enzymes. Acetylation is the addition of CH3CO functional group onto a compound. Although the physiological function of AAC(2’)-Ic is not certain, the discovery of the GNAT fold allowed researchers to classify AAC (2’)-Ic as an acetylating enzyme. Mycothiol is catalyzed by AAC (2’)-Ic to acetylate the aminoglycoside antibiotic known as tobramycin. When this occurs the aminoglycoside antibiotic becomes inactive. The basis of this study is important because when pathogens become resistant to commonly used antibiotics, an infection that was easily cured can now become severe and life threatening.[2]
| |||||||||||
References
- ↑ Vetting, M. W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin." RCSB Protien DataBase. N.p., 28 Aug.2002. Web. 13 July 2011. http://www.rcsb.org/pdb/explore/explore.do?structureId=1M4D
- ↑ 2.0 2.1 2.2 2.3 2.4 Vetting, Matthew W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin."Nature Structural Biology 9.9 (2002): 653-58. Print.
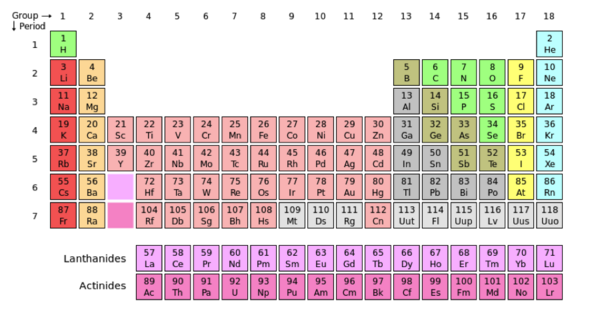
- ↑ User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov.2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.
- ↑ "Periodic Table." Wikipedia. Wikipedia, n.d. Web. 16 Nov. 2012.<http://en.wikipedia.org/wiki/Periodic_table>.
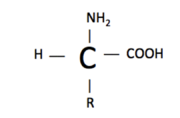
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Amino Acids." Wikipedia. N.p., n.d. Web. 13 Oct. 2012. <http://en.wikipedia.org/wiki/Amino_acid>.
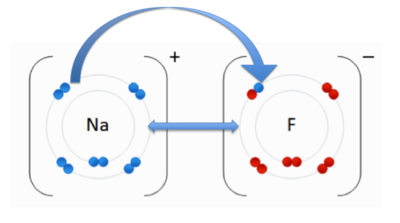
- ↑ . "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.
- ↑ User:Cepheus. "Ionic Bonds." Wikipedia. N.p., n.d. Web. 13 Nov. 2012. <http://en.wikipedia.org/wiki/Ionic_bond>.
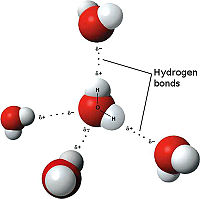
- ↑ Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.
- ↑ "Hydrogen Bond." wikipedia. N.p., n.d. Web. 4 Nov. 2012.<http://en.wikipedia.org/wiki/Hydrogen_bond>.
- ↑ "Wikipedia. N.p., n.d. Web. 12 Nov. 2012. <http://en.wikipedia.org/wiki/Protein_secondary_structure>.
- ↑ Wikipedia. Wikipedia, 4 Nov. 2012. Web. 7 Nov. 2012. <http://en.wikipedia.org/wiki/Enzyme_substrate_(biology)
- ↑ Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." "Coenzyme A." Wikipedia. N.p., n.d. Web. 11 Nov. 2012. <http://en.wikipedia.org/wiki/Coenzyme_A>.
- ↑ 13.0 13.1 13.2 13.3 "Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.