Hepatocyte growth factor receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | == | + | === Hepatocyte Growth Factor Receptor === |
| - | + | ||
| - | + | ||
| - | </StructureSection> | + | ==Introduction== |
| + | |||
| + | The Hepatocyte growth factor Receptor plays a large role in embryonic development, as its activation leads to events such as cell growth, motility and invasion. This receptor is a Tyrosine Kinase, and is one of the most well studied RTKs, as mutations in the c-met protooncogene can lead to tumorigenesis. The ligand for this receptor is Hepatocyte growth factor/scatter factor (HGF/SF), and upon binding of this ligand, the receptor becomes auto phosphorylated, causing downstream signaling events such as cell growth. <ref>PMID: 14559966</ref> C-Met is an αβ heterodimer with extracellular and intracellular domains. These two domains are disulfide linked together. <ref>PMID: 14559966</ref> | ||
| + | |||
| + | |||
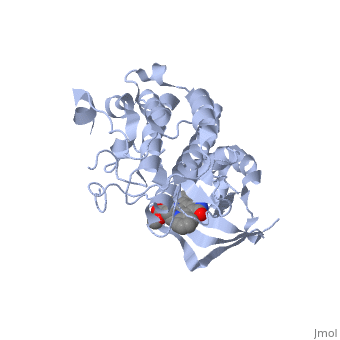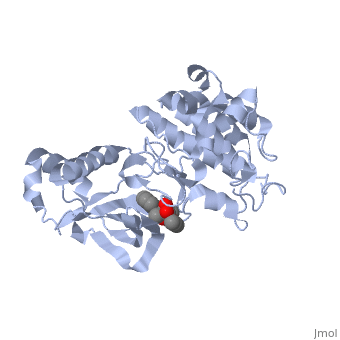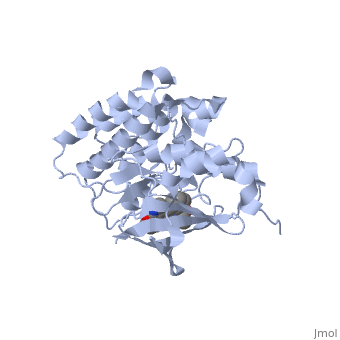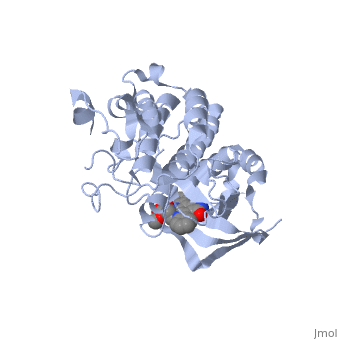
| + | <StructureSection load='1r1w' size='350' side='right' caption='Structure of Hepatocyte Growth Factor Receptor Tyrosine Kinase Domain (PDB entry [[1r1w]])' scene=''> | ||
| + | Some of the most important aspects of this RTK are the tyrosines at position 1234 and 1235. These two residues will become phosphorylated upon activation of the protein. There is another pair of important <scene name='User:Juliette_Personius/sandbox_1/Tyrosines_1349_and_1356/1'>tyrosine residues (1349 and 1356)</scene> . Studies with mice have shown that these tyrosines are necessary for normal c-met signaling. When these two tyrosines were substituted with with phenylalenine, the mice had an embryonically lethal phenotype and defects were found in placenta, liver, muscles and nerves. <ref>PMID: 8898205</ref> In a wild type c-met, these sites will become phosphorylated and act as docking sites for many different transducers and adapters. <ref>PMID: 14559966</ref> The c-met kinase domain is very similar to a typical protein kinase structure. | ||
| + | The N-terminal of the protein contains many <scene name='User:Juliette_Personius/sandbox_1/Beta_sheets/2'>β-sheets </scene> and is linked through a hinge to the C lobe, which is full of α helices. This particular kinase domain is very similar to the domains of the insulin receptor kinase and fibroblast growth factor receptor kinase. One main difference is that the c-met structure has an helix between residues 1060-1069 not present in FGFRK or IRK. <ref>PMID: 14559966</ref> | ||
| + | |||
| + | |||
| + | <StructureSection load='1r0p' size='350' side='right' caption='Structure of Hepatocyte Growth Factor Receptor Tyrosine Kinase Domain in complex with K-252a (PDB entry [[1r0p]])' scene=''> | ||
Revision as of 02:04, 4 December 2012
Hepatocyte Growth Factor Receptor
Introduction
The Hepatocyte growth factor Receptor plays a large role in embryonic development, as its activation leads to events such as cell growth, motility and invasion. This receptor is a Tyrosine Kinase, and is one of the most well studied RTKs, as mutations in the c-met protooncogene can lead to tumorigenesis. The ligand for this receptor is Hepatocyte growth factor/scatter factor (HGF/SF), and upon binding of this ligand, the receptor becomes auto phosphorylated, causing downstream signaling events such as cell growth. [1] C-Met is an αβ heterodimer with extracellular and intracellular domains. These two domains are disulfide linked together. [2]
| |||||||||||